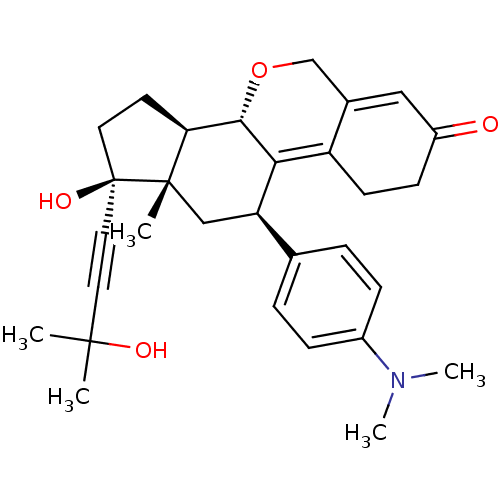
BDBM50200658 (8S,11R,13S,14R,17S)-11-(4-dimethylamino-phenyl)-17-hydroxy-17-(3-hydroxy-3-methyl-but-1-ynyl)-11,13-dimethyl-1,2,8,11,12,13,14,15,16,17-decahydro-6H-7-oxa-cyclopenta[a]phenanthren-3-one::CHEMBL231514
SMILES CN(C)c1ccc(cc1)[C@H]1C[C@@]2(C)[C@@H](CC[C@@]2(O)C#CC(C)(C)O)[C@@H]2OCC3=CC(=O)CCC3=C12
InChI Key InChIKey=GLKBGMLFLXJOJR-AAJJWQDLSA-N
Data 2 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50200658
Found 2 hits for monomerid = 50200658
TargetProgesterone receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: >1.00E+3nMAssay Description:Antagonist activity at progesterone receptor in human T47D cells assessed as inhibition of progesterone-induced alkaline phosphatase activityMore data for this Ligand-Target Pair
TargetGlucocorticoid receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 558nMAssay Description:Antagonist activity at glucocorticoid receptor in human A549 cells assessed as inhibition of corticoid-induced GRE-linked luciferase reporter gene ac...More data for this Ligand-Target Pair