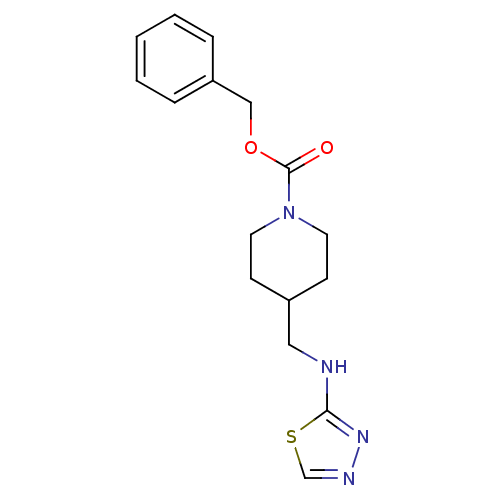
BDBM50203306 CHEMBL219113::benzyl 4-[(1,3,4-thiadiazol-2-ylamino)methyl]piperidine-1-carboxylate
SMILES O=C(OCc1ccccc1)N1CCC(CNc2nncs2)CC1
InChI Key InChIKey=JRLRJUXCVXQXRI-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50203306
Found 4 hits for monomerid = 50203306
TargetGlutamate receptor ionotropic, NMDA 2B(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 190nMAssay Description:Displacement of [3H](E)-N1-(2-methoxybenzyl)cinnamamidine from human NR2B expressed in Ltk- cellsMore data for this Ligand-Target Pair
Affinity DataIC50: >2.50E+4nMAssay Description:Inhibition of CYP2C9 in human liver microsomesMore data for this Ligand-Target Pair
Affinity DataIC50: >2.50E+4nMAssay Description:Inhibition of CYP3A4 in human liver microsomesMore data for this Ligand-Target Pair
Affinity DataIC50: >2.50E+4nMAssay Description:Inhibition of CYP2D6 in human liver microsomesMore data for this Ligand-Target Pair