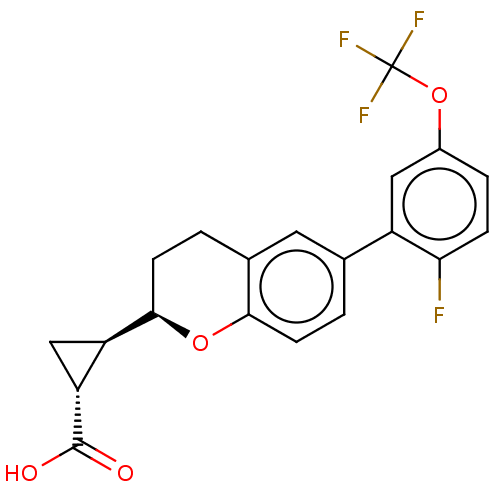
BDBM50207065 CHEMBL3971308
SMILES [H][C@]1(C[C@@]1([H])[C@@]1([H])CCc2cc(ccc2O1)-c1cc(OC(F)(F)F)ccc1F)C(O)=O
InChI Key InChIKey=WMWFFHNBBDQENV-IIDMSEBBSA-N
Data 3 EC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50207065
Found 3 hits for monomerid = 50207065
Affinity DataEC50: 69nMAssay Description:Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assayMore data for this Ligand-Target Pair
Affinity DataEC50: >1.00E+4nMAssay Description:Agonist activity at human GPR40 expressed in HEK293 cells assessed as increase in IP1 accumulation after 60 mins by HTRF assayMore data for this Ligand-Target Pair
Affinity DataEC50: 33nMAssay Description:Agonist activity at human GPR120 short isoform expressed in CHOK1 cells assessed as beta-arrestin recruitment after 90 mins by luminescence assayMore data for this Ligand-Target Pair