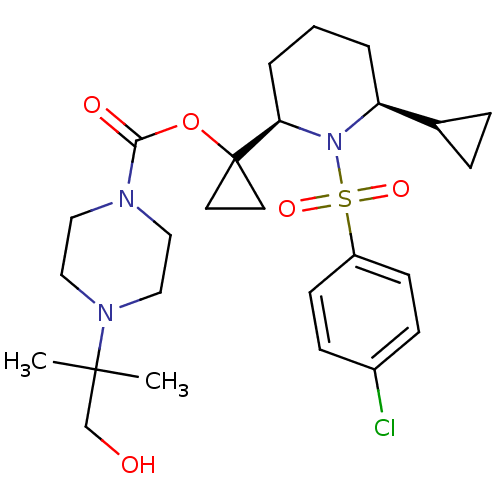
BDBM50220296 1-((2R,6S)-1-(4-chlorophenylsulfonyl)-6-cyclopropylpiperidin-2-yl)cyclopropyl 4-(1-hydroxy-2-methylpropan-2-yl)piperazine-1-carboxylate::CHEMBL393941
SMILES CC(C)(CO)N1CCN(CC1)C(=O)OC1(CC1)[C@H]1CCC[C@@H](C2CC2)N1S(=O)(=O)c1ccc(Cl)cc1
InChI Key InChIKey=QVNUOJRVQDGJQW-XZOQPEGZSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50220296
Found 4 hits for monomerid = 50220296
TargetAmyloid-beta precursor protein(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataIC50: 12nMAssay Description:Inhibition of gamma-secretase assessed as reduction of membrane Abeta40 levelMore data for this Ligand-Target Pair
Affinity DataIC50: 400nMAssay Description:Inhibition of CYP3A4 after 30 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 400nMAssay Description:Inhibition of CYP3A4 after 30 minsMore data for this Ligand-Target Pair
TargetAmyloid-beta precursor protein(Homo sapiens (Human))
Schering-Plough Research Institute
Curated by ChEMBL
Schering-Plough Research Institute
Curated by ChEMBL
Affinity DataIC50: 39nMAssay Description:Inhibition of gamma-secretase assessed as reduction of cell Abeta40 levelMore data for this Ligand-Target Pair