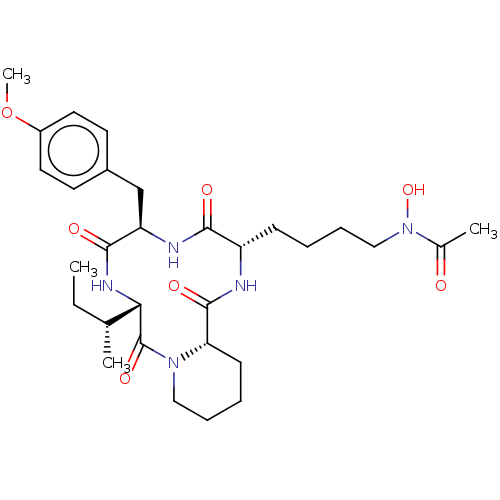
BDBM50221735 CHEMBL1790591
SMILES [H][C@@]12CCCCN1C(=O)[C@@]([H])(NC(=O)[C@@H](Cc1ccc(OC)cc1)NC(=O)[C@H](CCCCN(O)C(C)=O)NC2=O)[C@H](C)CC
InChI Key InChIKey=VAPCKGJQNDLKMF-GGJIVPGBSA-N
Data 1 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 1 hit for monomerid = 50221735
Found 1 hit for monomerid = 50221735
TargetHistone deacetylase 1/2/3/4/5/6/7/8/9/11/Polyamine deacetylase HDAC10(Mus musculus)
Institute Of Technology
Curated by ChEMBL
Institute Of Technology
Curated by ChEMBL
Affinity DataIC50: 8.85E+4nMAssay Description:Inhibitory activity against histone deacetylases (HDAC) prepared from mouse melanoma B16/BL6 cellsMore data for this Ligand-Target Pair