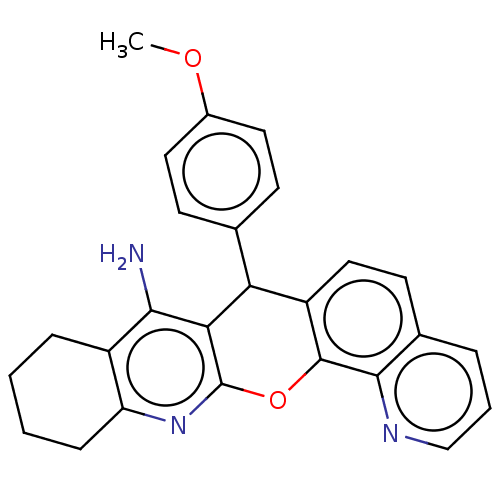
BDBM50234768 CHEMBL4085738
SMILES COc1ccc(cc1)C1c2ccc3cccnc3c2Oc2nc3CCCCc3c(N)c12
InChI Key InChIKey=UIYNBKZEXYKPIY-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50234768
Found 3 hits for monomerid = 50234768
Affinity DataIC50: 1.30E+3nMAssay Description:Inhibition of horse serum BuChE using butyrylthiocholine as substrate pretreated for 10 mins followed by substrate addition measured after 15 mins by...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
University Of Sfax
Curated by ChEMBL
University Of Sfax
Curated by ChEMBL
Affinity DataIC50: 40nMAssay Description:Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate pretreated for 10 mins followed by substrate addition measure...More data for this Ligand-Target Pair
Affinity DataIC50: 1.51E+3nMAssay Description:Inhibition of recombinant human AChE expressed in HEK293 cells using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrat...More data for this Ligand-Target Pair