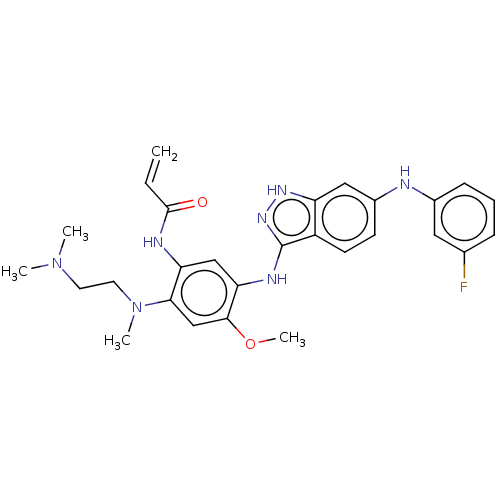
BDBM50237140 CHEMBL4068763
SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1n[nH]c2cc(Nc3cccc(F)c3)ccc12
InChI Key InChIKey=JLPBXDBUHXLBON-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50237140
Found 3 hits for monomerid = 50237140
Affinity DataKi: 5.10nMAssay Description:Reversible inhibition of wild-type human N-terminal GST-tagged EGFR cytoplasmic domain (669 to 1210 end amino acid residues) expressed in baculovirus...More data for this Ligand-Target Pair
Affinity DataEC50: 3.10E+3nMAssay Description:Inhibition of wild-type EGFR in human A431 cells assessed as reduction in cell viability after 96 hrs by CellTiterGlo assayMore data for this Ligand-Target Pair
Affinity DataIC50: <1nMAssay Description:In vitro Binding affinity towards 5-hydroxytryptamine 3 receptor was determinedMore data for this Ligand-Target Pair