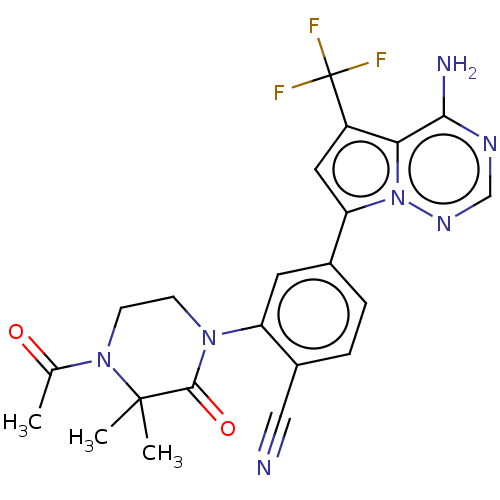
BDBM50239718 CHEMBL4064666::US10214537, Example 639
SMILES CC(=O)N1CCN(C(=O)C1(C)C)c1cc(ccc1C#N)-c1cc(c2c(N)ncnn12)C(F)(F)F
InChI Key InChIKey=ZDXIDBRGTYDHBQ-UHFFFAOYSA-N
Data 21 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 21 hits for monomerid = 50239718
Found 21 hits for monomerid = 50239718
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Bristol-Myers Squibb
US Patent
Bristol-Myers Squibb
US Patent
Affinity DataIC50: 2nMAssay Description:The ADP-Glo format PI3K assays were performed in Proxiplate 384-well plates (Perkin Elmer #6008280). The final assay volume was 2 μl prepared fr...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Mus musculus (Mouse))
Bristol-Myers Squibb
Curated by ChEMBL
Bristol-Myers Squibb
Curated by ChEMBL
Affinity DataIC50: 5.10nMAssay Description:Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by dibutyryl cyclic adenosine 3', 5...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Bristol-Myers Squibb
Curated by ChEMBL
Bristol-Myers Squibb
Curated by ChEMBL
Affinity DataIC50: 170nMAssay Description:Inhibition of PI3Kgamma (unknown origin) using PIP2:PS as substrate after 30 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
Bristol-Myers Squibb
Curated by ChEMBL
Bristol-Myers Squibb
Curated by ChEMBL
Affinity DataIC50: >5.00E+3nMAssay Description:Inhibition of PI3Kbeta (unknown origin) using PIP2:PS as substrate after 30 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Bristol-Myers Squibb
Curated by ChEMBL
Bristol-Myers Squibb
Curated by ChEMBL
Affinity DataIC50: 41nMAssay Description:Antibacterial activity against D-alanyl-D-alanine ligase from Streptococcus faecalis (ATCC 8043)More data for this Ligand-Target Pair
TargetMAP kinase-interacting serine/threonine-protein kinase 1(Homo sapiens (Human))
Bristol-Myers Squibb
Curated by ChEMBL
Bristol-Myers Squibb
Curated by ChEMBL
Affinity DataIC50: 66nMAssay Description:Inhibition of MNK1 (unknown origin) after 60 mins using fluorescein-labeled kinase tracer by HTRF assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Bristol-Myers Squibb
Curated by ChEMBL
Bristol-Myers Squibb
Curated by ChEMBL
Affinity DataIC50: 370nMAssay Description:Inhibition of PI3Kalpha (unknown origin) after 60 mins using fluorescein-labeled kinase tracer by HTRF assayMore data for this Ligand-Target Pair
TargetDual specificity protein kinase CLK4(Homo sapiens (Human))
Bristol-Myers Squibb
Curated by ChEMBL
Bristol-Myers Squibb
Curated by ChEMBL
Affinity DataIC50: 700nMAssay Description:Inhibition of CLK4 (unknown origin) after 60 mins using fluorescein-labeled kinase tracer by HTRF assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Bristol-Myers Squibb
US Patent
Bristol-Myers Squibb
US Patent
Affinity DataIC50: 1.70nMAssay Description:Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by exogenous histamineMore data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMAssay Description:Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by dibutyryl cyclic adenosine 3', 5...More data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMAssay Description:Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by exogenous histamineMore data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMAssay Description:Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by dibutyryl cyclic adenosine 3', 5...More data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMAssay Description:Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by dibutyryl cyclic adenosine 3', 5...More data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMAssay Description:Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by exogenous histamineMore data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMAssay Description:Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by dibutyryl cyclic adenosine 3', 5...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Bristol-Myers Squibb
US Patent
Bristol-Myers Squibb
US Patent
Affinity DataIC50: 35nMAssay Description:Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by exogenous histamineMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Bristol-Myers Squibb
Curated by ChEMBL
Bristol-Myers Squibb
Curated by ChEMBL
Affinity DataIC50: 1.20E+3nMAssay Description:Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by exogenous histamineMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Bristol-Myers Squibb
US Patent
Bristol-Myers Squibb
US Patent
Affinity DataIC50: <0.100nMAssay Description:Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by exogenous histamineMore data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMAssay Description:Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by exogenous histamineMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Bristol-Myers Squibb
US Patent
Bristol-Myers Squibb
US Patent
Affinity DataIC50: 35nMAssay Description:Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by exogenous histamineMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Bristol-Myers Squibb
US Patent
Bristol-Myers Squibb
US Patent
Affinity DataIC50: 2nMAssay Description:The ADP-Glo format PI3K assays were performed in Proxiplate 384-well plates (Perkin Elmer #6008280). The final assay volume was 2 μl prepared fr...More data for this Ligand-Target Pair