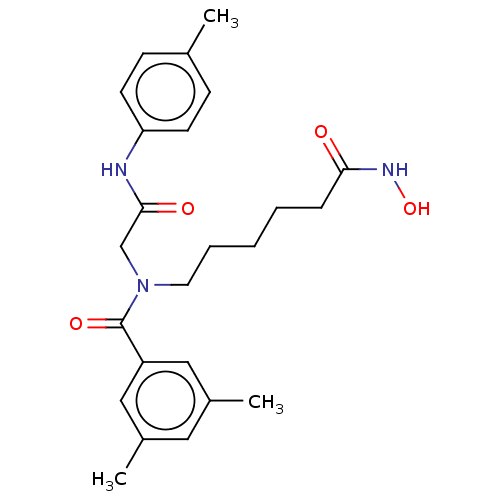
BDBM50239823 CHEMBL4098365
SMILES Cc1ccc(NC(=O)CN(CCCCCC(=O)NO)C(=O)c2cc(C)cc(C)c2)cc1
InChI Key InChIKey=BFKSCLUGYROMEJ-UHFFFAOYSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50239823
Found 4 hits for monomerid = 50239823
TargetHistone deacetylase 1/2/3/6(Homo sapiens (Human))
Heinrich-Heine-Universit£T D£Sseldorf
Curated by ChEMBL
Heinrich-Heine-Universit£T D£Sseldorf
Curated by ChEMBL
Affinity DataIC50: 810nMAssay Description:Inhibition of HDAC1/2/3/6 in human A2780cisR cells using Boc-Lys(epsilon-Ac)-AMC as substrate preincubated for 18 hrs followed by substrate addition ...More data for this Ligand-Target Pair
TargetHistone deacetylase 1/2/3/6(Homo sapiens (Human))
Heinrich-Heine-Universit£T D£Sseldorf
Curated by ChEMBL
Heinrich-Heine-Universit£T D£Sseldorf
Curated by ChEMBL
Affinity DataIC50: 950nMAssay Description:Inhibition of HDAC1/2/3/6 in human Cal27 cells using Boc-Lys(epsilon-Ac)-AMC as substrate preincubated for 18 hrs followed by substrate addition meas...More data for this Ligand-Target Pair
TargetHistone deacetylase 1/2/3/6(Homo sapiens (Human))
Heinrich-Heine-Universit£T D£Sseldorf
Curated by ChEMBL
Heinrich-Heine-Universit£T D£Sseldorf
Curated by ChEMBL
Affinity DataIC50: 800nMAssay Description:Inhibition of HDAC1/2/3/6 in human Cal27CisR cells using Boc-Lys(epsilon-Ac)-AMC as substrate preincubated for 18 hrs followed by substrate addition ...More data for this Ligand-Target Pair
TargetHistone deacetylase 1/2/3/6(Homo sapiens (Human))
Heinrich-Heine-Universit£T D£Sseldorf
Curated by ChEMBL
Heinrich-Heine-Universit£T D£Sseldorf
Curated by ChEMBL
Affinity DataIC50: 1.30E+3nMAssay Description:Inhibition of HDAC1/2/3/6 in human A2780 cells using Boc-Lys(epsilon-Ac)-AMC as substrate preincubated for 18 hrs followed by substrate addition meas...More data for this Ligand-Target Pair