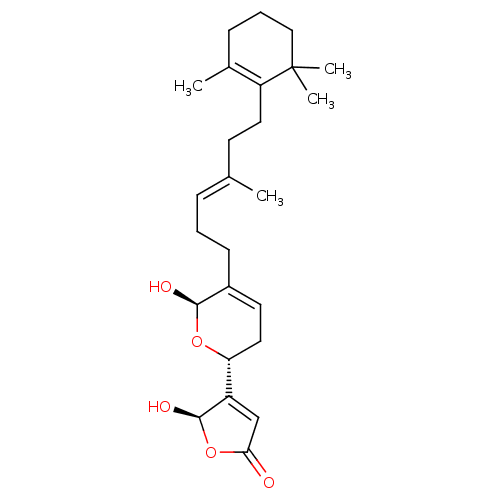
BDBM50250399 5-Hydroxy-4-{(R)-6-hydroxy-5-[(E)-4-methyl-6-(2,6,6-trimethyl-cyclohex-1-enyl)-hex-3-enyl]-3,6-dihydro-2H-pyran-2-yl}-5H-furan-2-one::5-Hydroxy-4-{6-hydroxy-5-[(E)-4-methyl-6-(2,6,6-trimethyl-cyclohex-1-enyl)-hex-3-enyl]-3,6-dihydro-2H-pyran-2-yl}-5H-furan-2-one::CHEMBL463914::manoalide
SMILES C\C(CCC1=C(C)CCCC1(C)C)=C/CCC1=CC[C@@H](O[C@H]1O)C1=CC(=O)O[C@H]1O
InChI Key InChIKey=FGJIDQWRRLDGDB-CPIXEKRISA-N
Data 21 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 21 hits for monomerid = 50250399
Found 21 hits for monomerid = 50250399
TargetAcidic phospholipase A2 2(Naja naja)
Istituto Per La Chimica Di Molecole Di Interesse Biologico Cnr
Curated by ChEMBL
Istituto Per La Chimica Di Molecole Di Interesse Biologico Cnr
Curated by ChEMBL
Affinity DataIC50: 1.70E+4nMAssay Description:Inhibition of Naja naja venom group1 sPLA2 at 10 uM by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 40nMAssay Description:Inhibition of bee venom PLA2More data for this Ligand-Target Pair
Affinity DataIC50: 7.50E+3nMAssay Description:Inhibition of bee venom group3 sPLA2 by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 3.90E+3nMAssay Description:Inhibition of human synovial recombinant group 2 secretory phospholipase A2 by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 7.50E+3nMAssay Description:Inhibition of bee venom group 3 secretory phospholipase A2 by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 400nMAssay Description:Inhibition of Crotalus adamanteus venom phospholipase A2 by fluorometric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 500nMAssay Description:Inhibition of bee venom phospholipase A2 after 5 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 500nMAssay Description:Inhibition of bee venom phospholipase A2 after 5 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+6nMAssay Description:In vitro inhibition of rat secretory Phospholipase A2 (group II).More data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+6nMAssay Description:In vitro inhibition of human recombinant secretory Phospholipase A2 (group II).More data for this Ligand-Target Pair
Affinity DataIC50: 7.50E+3nMAssay Description:Inhibitory activity against bee secretory Phospholipase A2 enzymeMore data for this Ligand-Target Pair
Affinity DataIC50: 3.90E+3nMAssay Description:Inhibitory activity against human synovial recombinant Phospholipase enzymeMore data for this Ligand-Target Pair
TargetDNA polymerase kappa(Homo sapiens (Human))
University Of Connecticut Health Center
Curated by ChEMBL
University Of Connecticut Health Center
Curated by ChEMBL
Affinity DataIC50: 3.40E+3nMAssay Description:Inhibition of human DNA polymerase kappa (19 to 526 residues) preincubated for 15 mins followed by replicating non-damaged DNA substrate addition mea...More data for this Ligand-Target Pair
TargetDNA polymerase kappa(Homo sapiens (Human))
University Of Connecticut Health Center
Curated by ChEMBL
University Of Connecticut Health Center
Curated by ChEMBL
Affinity DataIC50: 5.60E+3nMAssay Description:Inhibition of human DNA polymerase kappa (19 to 526 residues)-mediated TLS past acrolein derived ring-opened reduced form of gamma-HOPdG lesions prei...More data for this Ligand-Target Pair
Affinity DataIC50: 7.50E+3nMAssay Description:Inhibition of bee venom secretory PLA2More data for this Ligand-Target Pair
Affinity DataIC50: 3.90E+3nMAssay Description:Inhibition of human synovial secretory PLA2More data for this Ligand-Target Pair
Affinity DataIC50: 9.32E+4nMAssay Description:Inhibition of human synovial group2 sPLA2 at 10 uM by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 3.84E+4nMAssay Description:Inhibition of rat air pouch group2 sPLA2 at 10 uM by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 6.25E+4nMAssay Description:Inhibition of bee venom group3 sPLA2 at 10 uM by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 816nMAssay Description:Concentration required for in vitro inhibitory activity against human type II phospholipase A2More data for this Ligand-Target Pair
Affinity DataIC50: 3.90E+3nMAssay Description:Inhibition of human synovial group2 sPLA2 by liquid scintillation countingMore data for this Ligand-Target Pair