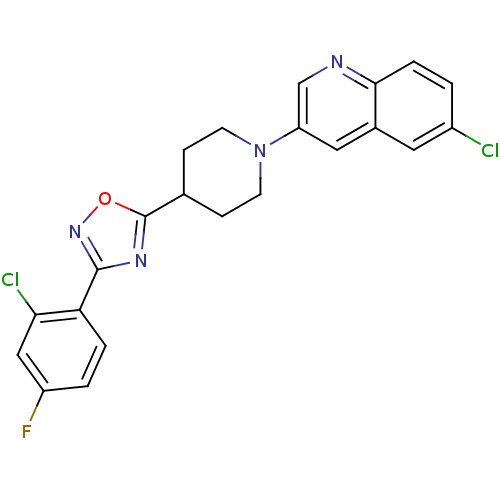
BDBM50261729 6-chloro-3-(4-(3-(2-chloro-4-fluorophenyl)-1,2,4-oxadiazol-5-yl)piperidin-1-yl)quinoline::CHEMBL468176
SMILES Fc1ccc(-c2noc(n2)C2CCN(CC2)c2cnc3ccc(Cl)cc3c2)c(Cl)c1
InChI Key InChIKey=KZPYBMNBUZPMKP-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 23 hits for monomerid = 50261729
Found 23 hits for monomerid = 50261729
Affinity DataEC50: 1.43E+3nMAssay Description:Agonist activity at human CB1 receptor expressed in insect Sf9 cells assessed as effect on Eu-GTP bindingMore data for this Ligand-Target Pair
Affinity DataEC50: 7nMAssay Description:Agonist activity at human CB2 receptor expressed in insect Sf9 cells assessed as effect on Eu-GTP bindingMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Amgen
Curated by ChEMBL
Amgen
Curated by ChEMBL
Affinity DataIC50: 500nMAssay Description:Displacement of radiolabeled dofetilide from human ERGMore data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of sst4 receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of adrenergic beta1 receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of dopamine D2 receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of 5HT1A receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of 5HT2B receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataEC50: >5.00E+4nMAssay Description:Agonist activity at sst4 receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of NK1 receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of mu opioid receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of histamine H1 receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of muscarinic M1 receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataEC50: >5.00E+4nMAssay Description:Agonist activity at adrenergic beta1 receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataEC50: >5.00E+4nMAssay Description:Agonist activity at dopamine D2 receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataEC50: >5.00E+4nMAssay Description:Agonist activity at 5HT1A receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataEC50: >5.00E+4nMAssay Description:Agonist activity at 5HT2B receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataEC50: >5.00E+4nMAssay Description:Agonist activity at NK1 receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataEC50: >5.00E+4nMAssay Description:Agonist activity at mu opioid receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataEC50: >5.00E+4nMAssay Description:Agonist activity at histamine H1 receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataEC50: >5.00E+4nMAssay Description:Agonist activity at muscarinic M1 receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataEC50: 11nMAssay Description:Agonist activity at human CB2 receptor assessed as inhibition of forskolin-induced increase in intracellular cAMPMore data for this Ligand-Target Pair
Affinity DataEC50: >2.00E+3nMAssay Description:Agonist activity at human CB1 receptor assessed as inhibition of forskolin-induced increase in intracellular cAMPMore data for this Ligand-Target Pair