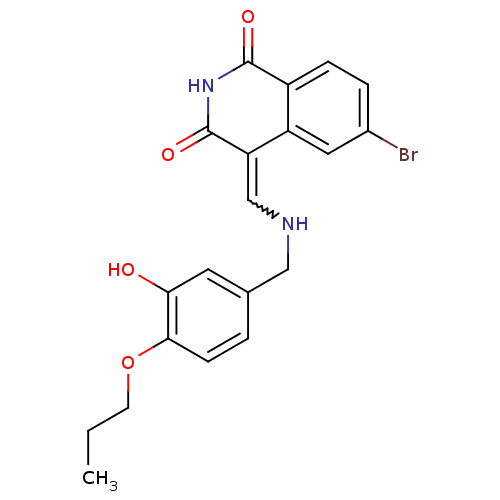
BDBM50267935 (4Z)-6-Bromo-4-{[(3-hydroxy-4propoxybenzyl)amino]methylene}-isoquinoline-1,3(2H,4H)-dione::CHEMBL485502
SMILES CCCOc1ccc(CNC=C2C(=O)NC(=O)c3ccc(Br)cc23)cc1O
InChI Key InChIKey=LCERHMDUMFVWTG-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50267935
Found 3 hits for monomerid = 50267935
Affinity DataIC50: <3.45E+4nMAssay Description:Inhibition of CDK2/Cyclin E (unknown origin) assessed as inhibition of retinoblastoma susceptibility gene product phosphorylationMore data for this Ligand-Target Pair
Affinity DataIC50: 350nMAssay Description:Inhibition of CDK4/Cyclin D1 (unknown origin) assessed as inhibition of retinoblastoma susceptibility gene product phosphorylationMore data for this Ligand-Target Pair
Affinity DataIC50: 4.25E+4nMAssay Description:Inhibition of CDK1/Cyclin B1 (unknown origin) assessed as inhibition of retinoblastoma susceptibility gene product phosphorylationMore data for this Ligand-Target Pair