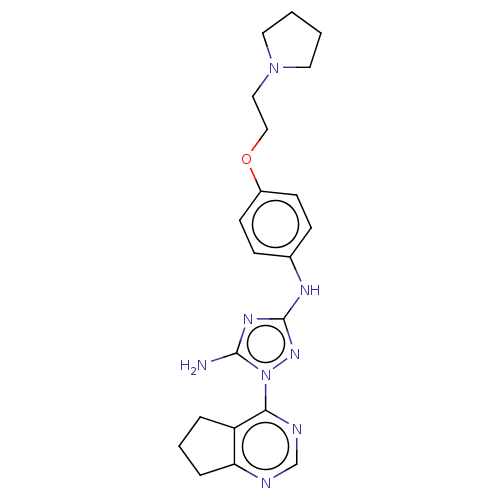
BDBM50269375 CHEMBL4086420
SMILES Nc1nc(Nc2ccc(OCCN3CCCC3)cc2)nn1-c1ncnc2CCCc12
InChI Key InChIKey=WAESMQNHFQFPCW-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50269375
Found 3 hits for monomerid = 50269375
TargetTyrosine-protein kinase receptor UFO(Homo sapiens (Human))
Rigel Pharmaceuticals
Curated by ChEMBL
Rigel Pharmaceuticals
Curated by ChEMBL
Affinity DataEC50: 23nMAssay Description:Inhibition of Axl in human HeLa cells assessed as reduction in AKT phosphorylation at Ser 473 residues pre-incubated for 1 hr before preclustered ant...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor UFO(Homo sapiens (Human))
Rigel Pharmaceuticals
Curated by ChEMBL
Rigel Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 14nMAssay Description:Inhibition of N-terminal GST-tagged human Axl (464 to 885 end residues) cytoplasmic domain expressed in baculovirus expression system using HS1 pepti...More data for this Ligand-Target Pair
Affinity DataEC50: 1.01E+3nMAssay Description:Inhibition of insulin stimulated INSR phosphorylation in human HeLa cells preincubated for 1 hr followed by insulin addition measured after 5 mins by...More data for this Ligand-Target Pair