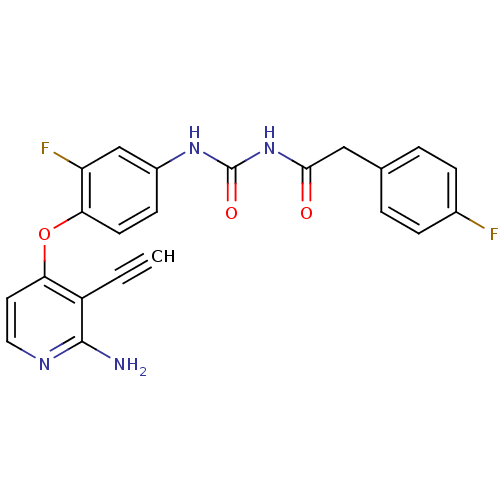
BDBM50272049 1-(4-(2-amino-3-ethynylpyridin-4-yloxy)-3-fluorophenyl)-3-(2-(4-fluorophenyl)acetyl)urea::CHEMBL502531
SMILES Nc1nccc(Oc2ccc(NC(=O)NC(=O)Cc3ccc(F)cc3)cc2F)c1C#C
InChI Key InChIKey=LKUSYUYBQDJFNA-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50272049
Found 3 hits for monomerid = 50272049
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of Met kinase (unknown origin)More data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of human c-METMore data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataIC50: 800nMAssay Description:Inhibition of human CYP3A4More data for this Ligand-Target Pair