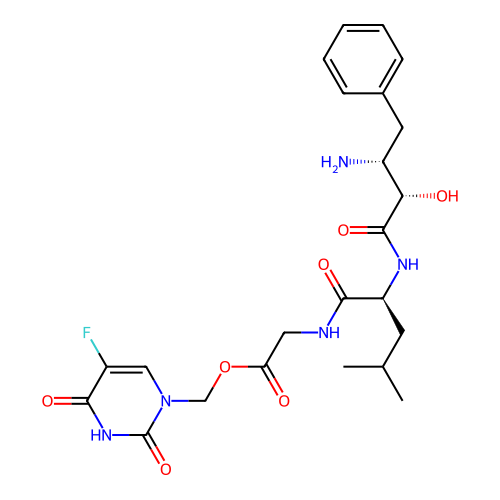
BDBM50284544 CHEMBL4163796
SMILES Cl.CC(C)C[C@H](NC(=O)[C@@H](O)[C@H](N)Cc1ccccc1)C(=O)NCC(=O)OCn1cc(F)c(=O)[nH]c1=O
InChI Key InChIKey=QVBSNIDCWLTTJC-UVXJWDRCSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50284544
Found 3 hits for monomerid = 50284544
Affinity DataIC50: 1.68E+3nMAssay Description:Inhibition of CD13 on surface of human ES2 cells using L-leucine-p-nitroanilide as substrate after 1 hrMore data for this Ligand-Target Pair
Affinity DataIC50: 180nMAssay Description:Inhibition of porcine kidney CD13 using L-leucine-p-nitroanilide as substrate preincubated for 5 mins followed by substrate addition measured after 3...More data for this Ligand-Target Pair
Affinity DataIC50: 5.69E+3nMAssay Description:Inhibition of CD13 on surface of human PLC/PRF/5 cells using L-leucine-p-nitroanilide as substrate after 1 hrMore data for this Ligand-Target Pair