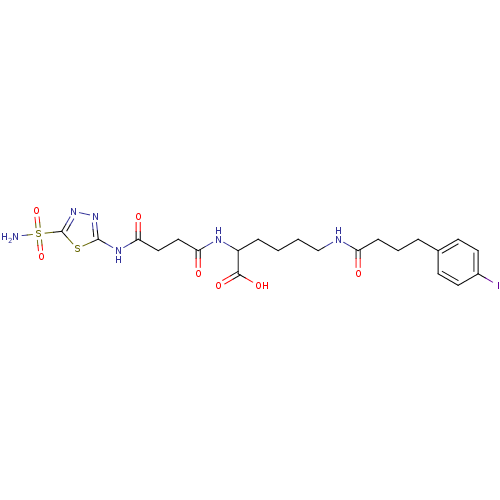
BDBM50298204 6-(4-(4-iodophenyl)butanamido)-2-(4-oxo-4-(5-sulfamoyl-1,3,4-thiadiazol-2-ylamino)butanamido)hexanoic acid::CHEMBL561947
SMILES NS(=O)(=O)c1nnc(NC(=O)CCC(=O)NC(CCCCNC(=O)CCCc2ccc(I)cc2)C(O)=O)s1
InChI Key InChIKey=MKYCTEHAGZSEMR-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50298204
Found 4 hits for monomerid = 50298204
TargetCarbonic anhydrase 9(Homo sapiens (Human))
Institute Of Pharmaceutical Sciences
Curated by ChEMBL
Institute Of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataKi: 8.80nMAssay Description:Inhibition of human CA9 catalytic domain using 4-nitrophenylacetate substrate in presence of murine serum albumin by colorimetryMore data for this Ligand-Target Pair
TargetCarbonic anhydrase 9(Homo sapiens (Human))
Institute Of Pharmaceutical Sciences
Curated by ChEMBL
Institute Of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataKi: 18nMAssay Description:Inhibition of human CA9 catalytic domain using 4-nitrophenylacetate substrate by colorimetryMore data for this Ligand-Target Pair
TargetCarbonic anhydrase 9(Homo sapiens (Human))
Institute Of Pharmaceutical Sciences
Curated by ChEMBL
Institute Of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataKd: 3.20nMAssay Description:Binding affinity to human CA9 catalytic domain by isothermal titration calorimetryMore data for this Ligand-Target Pair
Affinity DataKd: 820nMAssay Description:Binding affinity to HSA by isothermal titration calorimetryMore data for this Ligand-Target Pair