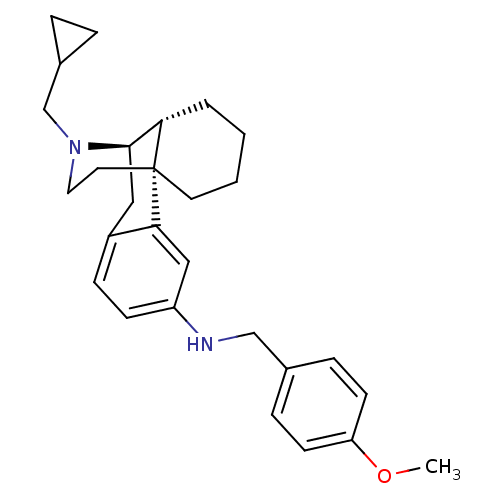
BDBM50303625 17-(Cyclopropylmethyl)-N-(4-methoxybenzyl)morphinan-3-amine::CHEMBL568877
SMILES COc1ccc(CNc2ccc3C[C@@H]4[C@@H]5CCCC[C@]5(CCN4CC4CC4)c3c2)cc1
InChI Key InChIKey=CHHRPKMGZRRHBF-KJYTXNCISA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 50303625
Found 9 hits for monomerid = 50303625
Affinity DataKi: 0.310nMAssay Description:Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.310nMAssay Description:Displacement of [3H]-DAMGO from human mu opioid receptor expressed in CHO cells after 60 mins by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 0.510nMAssay Description:Displacement of [3H]-U69,593 from human kappa opioid receptor expressed in CHO cells after 60 mins by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 0.510nMAssay Description:Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:Displacement of [3H]-naltrindole from human delta opioid receptor expressed in CHO cells after 3 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataEC50: 5.30nMAssay Description:Agonist activity at human kappa opioid receptor expressed in CHO cells assessed as stimulation of [33S]GTPgammaS binding after 60 mins by scintillati...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Antagonist activity at human mu opioid receptor expressed in CHO cells assessed as inhibition of DAMGO-induced [33S]GTPgammaS binding after 60 mins b...More data for this Ligand-Target Pair
Affinity DataEC50: 6.80nMAssay Description:Agonist activity at human mu opioid receptor expressed in CHO cells assessed as stimulation of [33S]GTPgammaS binding after 60 mins by scintillation ...More data for this Ligand-Target Pair