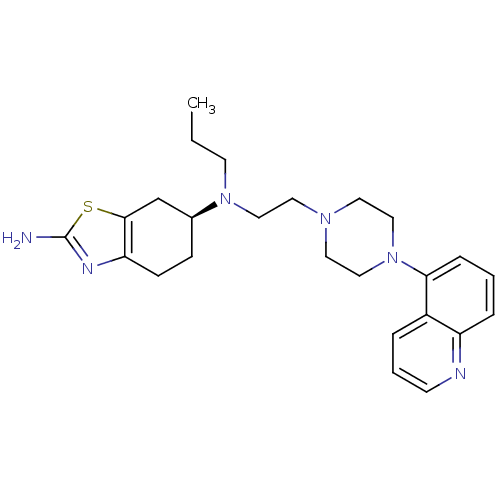
BDBM50303794 (-)-(S)-N6-Propyl-N6-(2-(4-(quinolin-5-yl)piperazin-1-yl)ethyl)-4,5,6,7-tetrahydrobenzo[d]thiazole-2,6-diamine::CHEMBL569107
SMILES CCCN(CCN1CCN(CC1)c1cccc2ncccc12)[C@H]1CCc2nc(N)sc2C1
InChI Key InChIKey=JULCETCXZSSJNN-IBGZPJMESA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50303794
Found 4 hits for monomerid = 50303794
Affinity DataKi: 1.21nMAssay Description:Displacement of [3H]spiperone from human dopamine D3 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 57.7nMAssay Description:Displacement of [3H]spiperone from human dopamine D2L receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataEC50: 13.5nMAssay Description:Agonist activity at human dopamine D2L receptor expressed in CHO cells assessed as stimulation of [35S]GTPgamma bindingMore data for this Ligand-Target Pair
Affinity DataEC50: 0.528nMAssay Description:Agonist activity at human dopamine D3 receptor expressed in mouse AtT-20 cells assessed as stimulation of [35S]GTPgamma bindingMore data for this Ligand-Target Pair