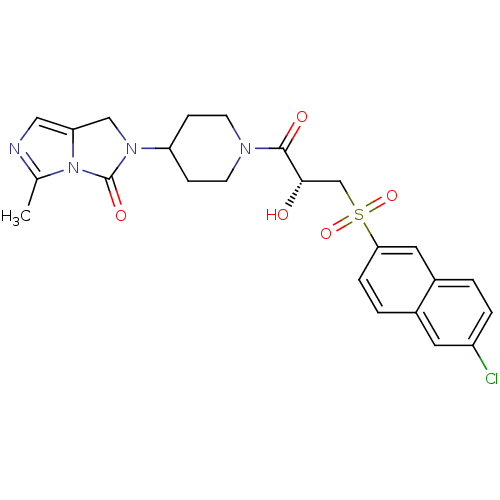
BDBM50304621 (R)-2-(1-(3-(6-chloronaphthalen-2-ylsulfonyl)-2-hydroxypropanoyl)piperidin-4-yl)-5-methyl-1H-imidazo[1,5-c]imidazol-3(2H)-one::2-(1-{(2R)-3-[(6-Chloronaphthalene-2-yl)sulfonyl]-2-hydroxypropanoyl}piperidin-4-yl)-5-methyl-1,2-dihydro-3Himidazo[1,5-c]imidazol::CHEMBL593236
SMILES Cc1ncc2CN(C3CCN(CC3)C(=O)[C@@H](O)CS(=O)(=O)c3ccc4cc(Cl)ccc4c3)C(=O)n12
InChI Key InChIKey=WSDPDXBHHQAFEA-QFIPXVFZSA-N
Data 2 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50304621
Found 2 hits for monomerid = 50304621
Affinity DataIC50: 21nMAssay Description:Inhibition of human factor 10a by para-nitroanilide release assayMore data for this Ligand-Target Pair
Affinity DataIC50: 21nMAssay Description:Inhibition of human factor 10a assessed as p-nitroanilide release using S2765 as substrate by chromogenic assayMore data for this Ligand-Target Pair