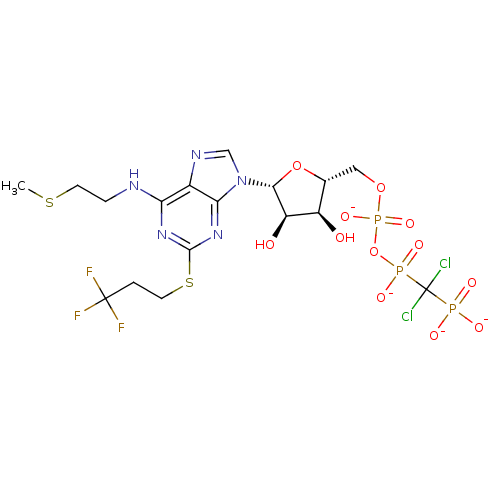
BDBM50318031 CHEMBL1097279::cangrelor
SMILES CSCCNc1nc(SCCC(F)(F)F)nc2n(cnc12)[C@@H]1O[C@H](COP([O-])(=O)OP([O-])(=O)C(Cl)(Cl)P([O-])([O-])=O)[C@@H](O)[C@H]1O
InChI Key InChIKey=PAEBIVWUMLRPSK-UHFFFAOYSA-J
Data 2 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50318031
Found 2 hits for monomerid = 50318031
Affinity DataIC50: 0.700nMAssay Description:Antagonist activity at P2Y12 receptor by [35S]GTPgammaS binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:Antagonist activity at human GPR17 expressed in human 1321N1 cells assessed as inhibition of UDP-glucose-induced [35S]GTPgammaS binding after 30 mins...More data for this Ligand-Target Pair