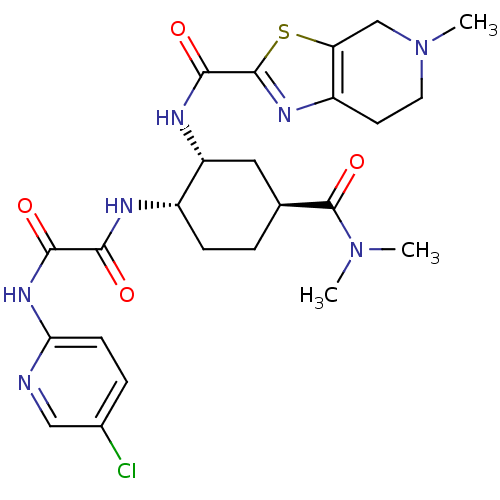
BDBM50328731 CHEMBL1269025::Edoxaban
SMILES CN(C)C(=O)[C@H]1CC[C@H](NC(=O)C(=O)Nc2ccc(Cl)cn2)[C@@H](C1)NC(=O)c1nc2CCN(C)Cc2s1
InChI Key InChIKey=HGVDHZBSSITLCT-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 50328731
Found 8 hits for monomerid = 50328731
Affinity DataKi: 0.560nMAssay Description:Inhibition of factor 10aMore data for this Ligand-Target Pair
Affinity DataKi: 0.561nMAssay Description:Inhibition of factor Xa (unknown origin) assessed as inhibition constantMore data for this Ligand-Target Pair
Affinity DataKi: 0.561nMAssay Description:Binding affinity to FXa (unknown origin) assessed as inhibition constantMore data for this Ligand-Target Pair
Affinity DataKi: 0.561nMAssay Description:Inhibition of activated human factor XMore data for this Ligand-Target Pair
TargetPlatelet-derived growth factor receptor alpha/beta(Mouse)
Zhengzhou University
Curated by ChEMBL
Zhengzhou University
Curated by ChEMBL
Affinity DataKi: 3nMAssay Description:Inhibition of activated human factor X/activated human factor V complex derived prothrombinase using S-2238 as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 3.40nMAssay Description:Inhibition of human factor Xa using Z-D-Arg-Gly-Arg-pNA.2HCl as substrate preincubated for 15 mins followed by substrate addition by UV absorption an...More data for this Ligand-Target Pair
Affinity DataIC50: 3.40nMAssay Description:Inhibition of human coagulation factor 10a using Z-D-Arg-Gly-Arg-pNA.2HCl as substrate preincubated for 15 mins followed by substrate addition by UV ...More data for this Ligand-Target Pair
Affinity DataKi: 6.00E+3nMAssay Description:Binding affinity to thrombin (unknown origin) assessed as inhibition constantMore data for this Ligand-Target Pair