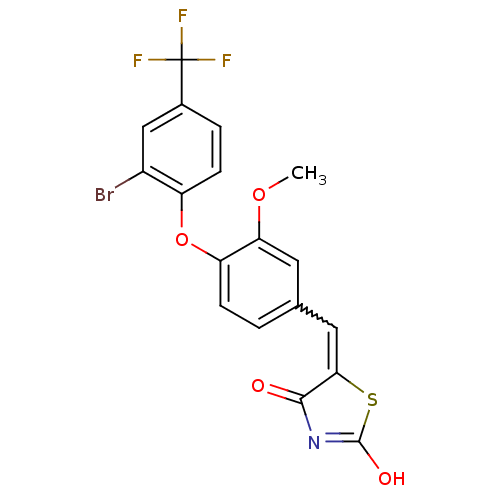
BDBM50336757 5-[4-(2-Bromo-4-trifluoromethylphenoxy)-3-methoxybenzylidene]thiazolidine-2,4-dione::CHEMBL1671967
SMILES COc1cc(C=C2SC(O)=NC2=O)ccc1Oc1ccc(cc1Br)C(F)(F)F
InChI Key InChIKey=PMEAQKGKOGFUOS-UHFFFAOYSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50336757
Found 4 hits for monomerid = 50336757
TargetSteroid hormone receptor ERR1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 140nMAssay Description:Antagonist activity at 6his-tagged ERRalpha LBD assessed as inhibition of recruitment of GST-labeled coactivator Scr2 by TR-FRET assayMore data for this Ligand-Target Pair
TargetSteroid hormone receptor ERR1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 2.70E+3nMAssay Description:Antagonist activity at ERRalpha LBD expressed in HEK293 cells assessed as Gal4-SRC2 interaction by two hybrid luciferase reporter gene assayMore data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 3.80E+3nMAssay Description:Inhibition of CYP3A4More data for this Ligand-Target Pair
TargetCytochrome P450 2D6(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of CYP2D6More data for this Ligand-Target Pair