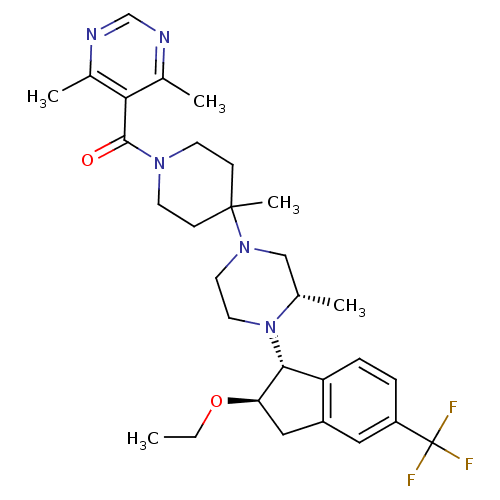
BDBM50339033 5-[(4-(3S)-4-[(1R,2R)-2-ethoxy-5-(trifluoromethyl)-2,3-dihydro-1H-inden-1-yl]-3-methylpiperazin-1-yl-4-methylpiperidin-1-yl)carbonyl]-4,6-dimethylpyrimidine::CHEMBL1688243
SMILES CCO[C@@H]1Cc2cc(ccc2[C@H]1N1CCN(C[C@@H]1C)C1(C)CCN(CC1)C(=O)c1c(C)ncnc1C)C(F)(F)F
InChI Key InChIKey=ZMCJFJZOSKEMOM-DNKZPPIMSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 13 hits for monomerid = 50339033
Found 13 hits for monomerid = 50339033
Affinity DataIC50: 6.5nMAssay Description:Displacement of [125I]MIP-1beta from CCR5 in IL-10-stimulated human monocytesMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Antagonist activity at CCR5 in IL-10 stimulated human PBMC cells assessed as MIP-1beta induced chemotaxisMore data for this Ligand-Target Pair
Affinity DataKd: 3.10nMAssay Description:Activation of CCR5 in human PBMC cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Antagonist activity at CCR5 assessed as inhibition of intracellular calcium mobilizationMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Antagonist activity at CCR5 assessed as inhibition of ERK phosphorylationMore data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:Antagonist activity at CCR5 assessed as inhibition of receptor internalizationMore data for this Ligand-Target Pair
Affinity DataIC50: 3.10nMAssay Description:Binding affinity to CCR5More data for this Ligand-Target Pair
Affinity DataIC50: >2.50E+4nMAssay Description:Inhibition of CYP3A4More data for this Ligand-Target Pair
Affinity DataIC50: >2.50E+4nMAssay Description:Inhibition of CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: >2.50E+4nMAssay Description:Inhibition of CYP1A2More data for this Ligand-Target Pair
Affinity DataIC50: >2.50E+4nMAssay Description:Inhibition of CYP2C9More data for this Ligand-Target Pair
Affinity DataIC50: >2.50E+4nMAssay Description:Inhibition of CYP2C19More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 4.50E+3nMAssay Description:Inhibition of human hERGMore data for this Ligand-Target Pair