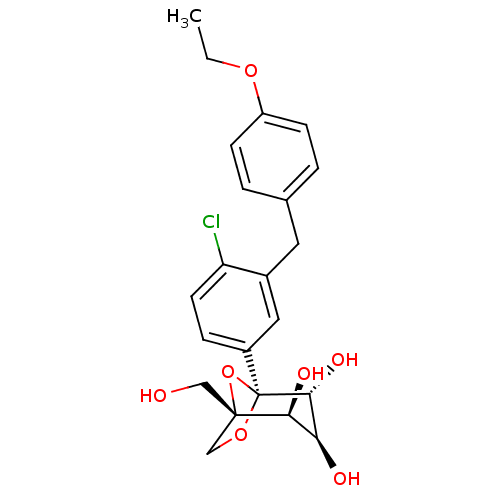
BDBM50342885 (1S,2S,3S,4R,5S)-5-[4-Chloro-3-(4-ethoxybenzyl)phenyl]-1-(hydroxymethyl)-6,8-dioxa-bicyclo[3.2.1]octane-2,3,4-triol::5-(4-chloro-3-(4-ethoxybenzyl)phenyl)-1-hydroxymethyl-6,8-dioxabicyclo(3.2.1)octane-2,3,4-triol::CHEMBL1770248::PF-04971729::Steglatro::ertugliflozin
SMILES CCOc1ccc(Cc2cc(ccc2Cl)[C@]23OC[C@](CO)(O2)[C@@H](O)[C@H](O)[C@H]3O)cc1
InChI Key InChIKey=MCIACXAZCBVDEE-UHFFFAOYSA-N
Data 9 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 50342885
Found 9 hits for monomerid = 50342885
Affinity DataIC50: 0.877nMAssay Description:Inhibition of human SGLT2 expressed in CHO cells assessed as inhibition of methyl alpha-D-glucopyranoside uptake after 1 hrMore data for this Ligand-Target Pair
Affinity DataIC50: 0.877nMAssay Description:Inhibition of human SGLT2More data for this Ligand-Target Pair
Affinity DataIC50: 0.880nMAssay Description:Inhibition of human SGLT2More data for this Ligand-Target Pair
Affinity DataIC50: 1.15nMAssay Description:Inhibition of rat SGLT2More data for this Ligand-Target Pair
Affinity DataIC50: 2.40nMAssay Description:Inhibition of human SGLT2 expressed in CHO cells assessed as decrease in uptake of [14C]AMG after 120 mins by TopCount methodMore data for this Ligand-Target Pair
Affinity DataIC50: 392nMAssay Description:Inhibition of human SGLT1 expressed in CHO cells assessed as decrease in uptake of [14C]AMG after 120 mins by TopCount methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.96E+3nMAssay Description:Inhibition of human SGLT1 expressed in CHO cells assessed as inhibition of methyl alpha-D-glucopyranoside uptake after 1 hrMore data for this Ligand-Target Pair
Affinity DataIC50: 1.96E+3nMAssay Description:Inhibition of human SGLT1More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of human SGLT1More data for this Ligand-Target Pair