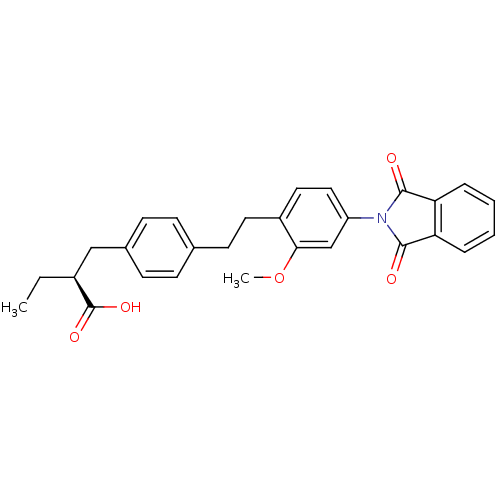
BDBM50349834 CHEMBL1813200
SMILES CC[C@@H](Cc1ccc(CCc2ccc(cc2OC)N2C(=O)c3ccccc3C2=O)cc1)C(O)=O
InChI Key InChIKey=CYBICBBHMXTCRI-FQEVSTJZSA-N
Data 3 EC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50349834
Found 3 hits for monomerid = 50349834
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
The University Of Tokyo
Curated by ChEMBL
The University Of Tokyo
Curated by ChEMBL
Affinity DataEC50: 7.10E+3nMAssay Description:Agonist activity at PPARgamma expressed in HEK293 after 8 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor alpha(Homo sapiens (Human))
The University Of Tokyo
Curated by ChEMBL
The University Of Tokyo
Curated by ChEMBL
Affinity DataEC50: 8.90E+3nMAssay Description:Agonist activity at human PPARalpha expressed in HEK293 after 8 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor delta(Homo sapiens (Human))
The University Of Tokyo
Curated by ChEMBL
The University Of Tokyo
Curated by ChEMBL
Affinity DataEC50: 7.30E+3nMAssay Description:Agonist activity at PPARdelta expressed in HEK293 after 8 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair