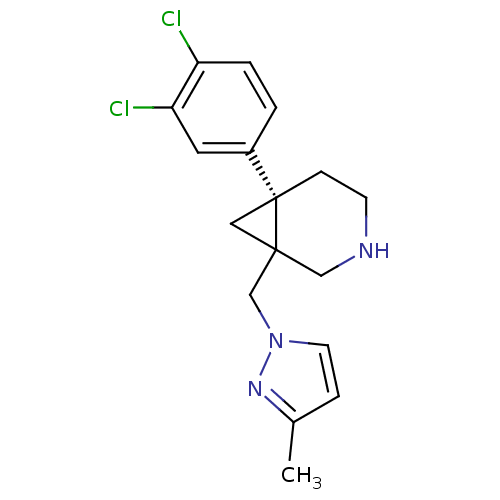
BDBM50350764 CHEMBL1818451
SMILES Cc1ccn(CC23C[C@]2(CCNC3)c2ccc(Cl)c(Cl)c2)n1
InChI Key InChIKey=VUEZBOYAXGKAME-ZYMOGRSISA-N
Data 5 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 50350764
Found 5 hits for monomerid = 50350764
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human CYP1A2 incubated for 10 mins before substrate addition by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human CYP2C9 incubated for 10 mins before substrate addition by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human CYP3A4 incubated for 10 mins before substrate addition by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human CYP2D6 incubated for 10 mins before substrate addition by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human CYP2C19 incubated for 10 mins before substrate addition by fluorescence assayMore data for this Ligand-Target Pair