BDBM50353128 CHEMBL1231795
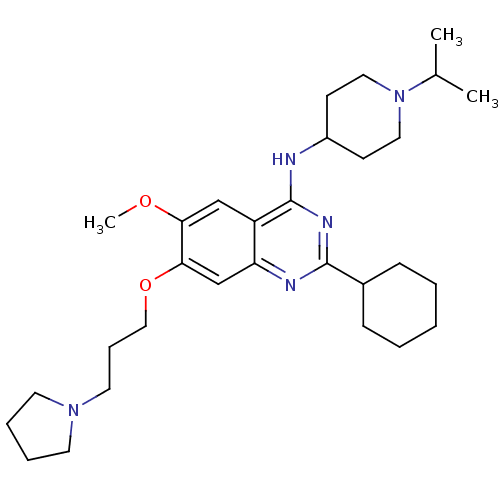
SMILES COc1cc2c(NC3CCN(CC3)C(C)C)nc(nc2cc1OCCCN1CCCC1)C1CCCCC1
InChI Key InChIKey=QOECJCJVIMVJGX-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50353128
Found 3 hits for monomerid = 50353128
TargetHistone-lysine N-methyltransferase EHMT2(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataKi: 3.70nMAssay Description:Competitive inhibition of G9a (unknown origin) by Morrison plot analysis in presence of histone H3 (1 to 25 residues)More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EHMT2(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 610nMAssay Description:Inhibition of G9a (unknown origin) using biotinylated-histone H3 (1 to 21 residues)/S-adenosyl-methionine as substrate/methyl donor after 3 hrs by Al...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EHMT2(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: <15nMAssay Description:Inhibition of G9a (unknown origin) using histone H3 (1 to 25 residues) as substrate preincubated for 2 mins followed by substrate addition measured f...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)