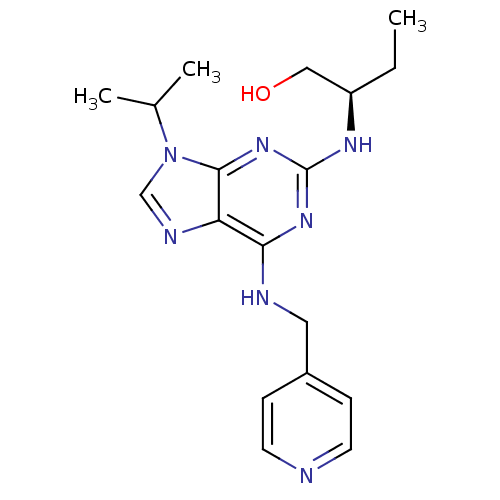
BDBM50358224 CHEMBL455641
SMILES CC[C@H](CO)Nc1nc(NCc2ccncc2)c2ncn(C(C)C)c2n1
InChI Key InChIKey=BJDIQUJXLIZDRH-CQSZACIVSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50358224
Found 4 hits for monomerid = 50358224
TargetCyclin-H/Cyclin-dependent kinase 7(Homo sapiens (Human))
The Institute Of Cancer Research
Curated by ChEMBL
The Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 740nMAssay Description:Inhibition of CDK7/cyclin HMore data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta/[Tau protein] kinase(Sus scrofa)
Universite Paris-Descartes
Curated by ChEMBL
Universite Paris-Descartes
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of pig brain GSK3alpha/betaMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase/G2/mitotic-specific cyclin- 1(Homo sapiens (Human))
The Institute Of Cancer Research
Curated by ChEMBL
The Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 2.80E+4nMAssay Description:Inhibition of CDK1/cyclin BMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 1(Homo sapiens (Human))
The Institute Of Cancer Research
Curated by ChEMBL
The Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 3.20E+4nMAssay Description:Inhibition of ERK2More data for this Ligand-Target Pair