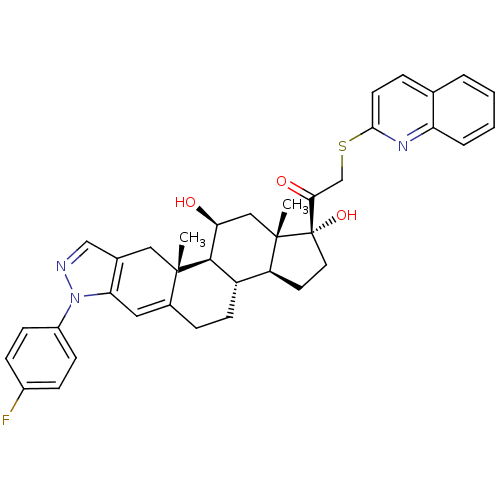
BDBM50381731 CHEMBL2023614
SMILES C[C@]12C[C@H](O)[C@H]3[C@@H](CCC4=Cc5c(C[C@]34C)cnn5-c3ccc(F)cc3)[C@@H]1CC[C@]2(O)C(=O)CSc1ccc2ccccc2n1
InChI Key InChIKey=ZBDBTKZKRVQZDY-BKRLQIJFSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50381731
Found 3 hits for monomerid = 50381731
Affinity DataIC50: 18.3nMAssay Description:Transactivation activity at glucocorticoid receptor in human HepG2 cells assessed as induction of tyrosineaminotransferaseMore data for this Ligand-Target Pair
Affinity DataIC50: 5.30nMAssay Description:Transrepression activity at glucocorticoid receptor in human H292 cells assessed as inhibition of TNF-induced IL8 productionMore data for this Ligand-Target Pair
Affinity DataIC50: 24.3nMAssay Description:Transactivation activity at glucocorticoid receptor in rat H4II-E cells assessed as induction of tyrosineaminotransferaseMore data for this Ligand-Target Pair