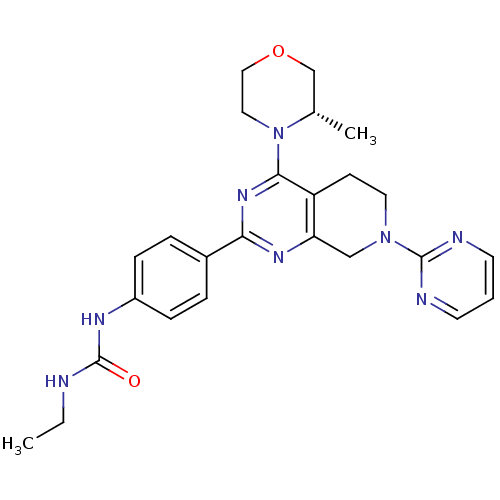
BDBM50400338 CHEMBL2181522
SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2CN(CCc2c(n1)N1CCOC[C@@H]1C)c1ncccn1
InChI Key InChIKey=WQBAZXIQANTUOY-KRWDZBQOSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50400338
Found 3 hits for monomerid = 50400338
Affinity DataKi: 1.5nMAssay Description:Inhibition of recombinant mTOR (1360 to 2549)+GBL (unknown origin) using GFP-4E-BP1 as substrate after 30 mins by FRET assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.5nMAssay Description:Inhibition of human recombinant mTOR by FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 900nMAssay Description:Time-dependent inhibition of CYP3A4 in human liver microsome using midazolam as substrate by TDI shift assayMore data for this Ligand-Target Pair