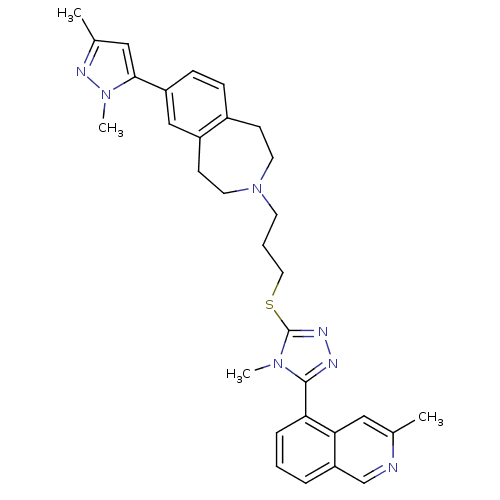
BDBM50411678 CHEMBL273199
SMILES Cc1cc(-c2ccc3CCN(CCCSc4nnc(-c5cccc6cnc(C)cc56)n4C)CCc3c2)n(C)n1
InChI Key InChIKey=RLZDEABBOHACIP-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50411678
Found 4 hits for monomerid = 50411678
Affinity DataKi: 1.58nMAssay Description:Antagonist activity at human dopamine D3 receptor by cell based GTPgammaS binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 316nMAssay Description:Antagonist activity at dopamine D2 receptor by GTPgammaS binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 794nMAssay Description:Antagonist activity at histamine H1 receptor by FLIPR assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Displacement of [3H]Dofetilide from human ERGMore data for this Ligand-Target Pair