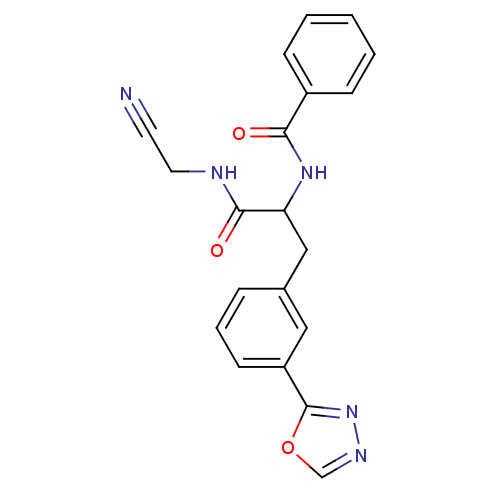
BDBM50414635 CHEMBL564626
SMILES O=C(NCC#N)C(Cc1cccc(c1)-c1nnco1)NC(=O)c1ccccc1
InChI Key InChIKey=ZRKTXOUQLJMAQT-UHFFFAOYSA-N
Data 5 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 50414635
Found 5 hits for monomerid = 50414635
Affinity DataIC50: 501nMAssay Description:Inhibition of cathepsin L assessed as inhibition of fluorogenic substrate cleavageMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of human liver cathepsin B assessed as inhibition of fluorogenic substrate cleavageMore data for this Ligand-Target Pair
Affinity DataIC50: 19.9nMAssay Description:Inhibition of cathepsin S assessed as inhibition of fluorogenic substrate cleavageMore data for this Ligand-Target Pair
Affinity DataIC50: 158nMAssay Description:Inhibition of cathepsin L2 assessed as inhibition of fluorogenic substrate cleavageMore data for this Ligand-Target Pair
Affinity DataIC50: 1.26E+3nMAssay Description:Inhibition of cathepsin K assessed as inhibition of fluorogenic substrate cleavageMore data for this Ligand-Target Pair