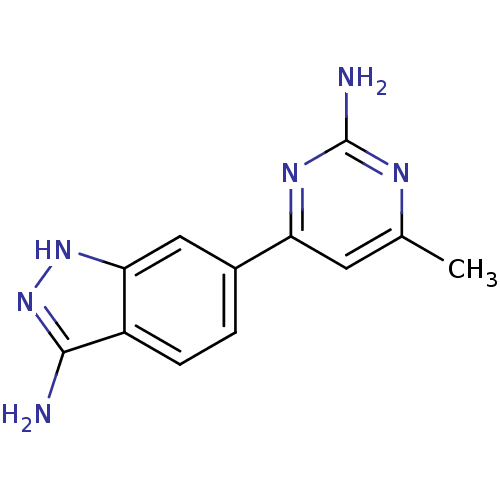
BDBM50418259 CHEMBL1765756
SMILES Cc1cc(nc(N)n1)-c1ccc2c(N)n[nH]c2c1
InChI Key InChIKey=JZOJBOMGNJTZMY-UHFFFAOYSA-N
Data 5 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 50418259
Found 5 hits for monomerid = 50418259
Affinity DataIC50: 3.98E+3nMAssay Description:Inhibition of ROCK1More data for this Ligand-Target Pair
Target3-phosphoinositide-dependent protein kinase 1(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 126nMAssay Description:Inhibition of PDK1-mediated AKT phosphorylation at Thr308 residue in human PC3 cells by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 7.94E+3nMAssay Description:Inhibition of ALK5More data for this Ligand-Target Pair
Affinity DataIC50: 1.26E+4nMAssay Description:Inhibition of aurora BMore data for this Ligand-Target Pair
Affinity DataIC50: 3.98E+3nMAssay Description:Inhibition of aurora AMore data for this Ligand-Target Pair