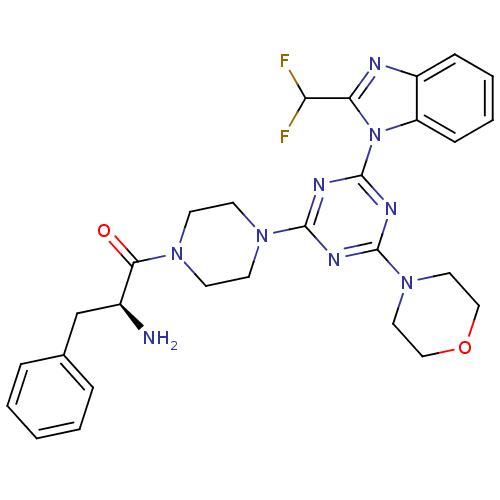
BDBM50426450 CHEMBL2322664
SMILES N[C@@H](Cc1ccccc1)C(=O)N1CCN(CC1)c1nc(nc(n1)-n1c(nc2ccccc12)C(F)F)N1CCOCC1
InChI Key InChIKey=VOPWCDJBVPMVLJ-FQEVSTJZSA-N
Data 8 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 50426450
Found 8 hits for monomerid = 50426450
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
Monash University
Curated by ChEMBL
Monash University
Curated by ChEMBL
Affinity DataIC50: 2.80E+3nMAssay Description:Inhibition of PI3Kbeta in human platelet-rich plasma assessed as suppression of ADP-induced platelet aggregation preincubated for 5 mins followed by ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
Monash University
Curated by ChEMBL
Monash University
Curated by ChEMBL
Affinity DataIC50: 30nMAssay Description:Inhibition of PI3Kbeta in human washed platelets assessed as suppression of ADP-induced platelet aggregation preincubated for 5 mins followed by ADP ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataIC50: 2.20E+3nMAssay Description:Inhibition of PI3K-delta (unknown origin) assessed as decrease in ATP consumption using phosphotidylinositol bisphosphate and 10 uM ATP as substrate ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataIC50: 1.20E+4nMAssay Description:Inhibition of wild type PI3K-alpha (unknown origin) assessed as decrease in ATP consumption using phosphotidylinositol bisphosphate and 100 uM ATP as...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
Monash University
Curated by ChEMBL
Monash University
Curated by ChEMBL
Affinity DataIC50: 63nMAssay Description:Inhibition of PI3K-beta (unknown origin) assessed as decrease in ATP consumption using phosphotidylinositol bisphosphate and 10 uM ATP as substrate m...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataIC50: 4.70E+3nMAssay Description:Inhibition of PI3K-alpha (unknown origin) assessed as decrease in ATP consumption using phosphotidylinositol bisphosphate and 10 uM ATP as substrate ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
Monash University
Curated by ChEMBL
Monash University
Curated by ChEMBL
Affinity DataIC50: 74nMAssay Description:Inhibition of wild type PI3K-beta (unknown origin) assessed as decrease in ATP consumption using phosphotidylinositol bisphosphate and 100 uM ATP as ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Monash University (Parkville Campus)
Curated by ChEMBL
Monash University (Parkville Campus)
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of PI3K-gamma (unknown origin) assessed as decrease in ATP consumption using phosphotidylinositol bisphosphate and 10 uM ATP as substrate ...More data for this Ligand-Target Pair