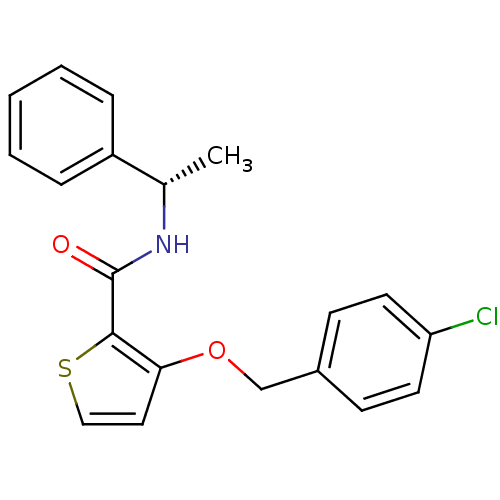
BDBM50430584 CHEMBL2337806
SMILES C[C@H](NC(=O)c1sccc1OCc1ccc(Cl)cc1)c1ccccc1
InChI Key InChIKey=RFZPGNRLOKVZJY-AWEZNQCLSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50430584
Found 3 hits for monomerid = 50430584
TargetPhosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2(Homo sapiens (Human))
University Of East Anglia
Curated by ChEMBL
University Of East Anglia
Curated by ChEMBL
Affinity DataKi: 440nMAssay Description:Inhibition of human SHIP2 (419 to 732 residues) expressed in Escherichia coli by malachite green phosphate assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2(Homo sapiens (Human))
University Of East Anglia
Curated by ChEMBL
University Of East Anglia
Curated by ChEMBL
Affinity DataIC50: 620nMAssay Description:Inhibition of SHIP2 (unknown origin)More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2(Homo sapiens (Human))
University Of East Anglia
Curated by ChEMBL
University Of East Anglia
Curated by ChEMBL
Affinity DataIC50: 1.50E+3nMAssay Description:Inhibition of human SHIP2 catalytic domain (419 to 832 residues) phosphatase activity assessed as phosphate release using Ins(1,3,4,5)P4 as substrate...More data for this Ligand-Target Pair