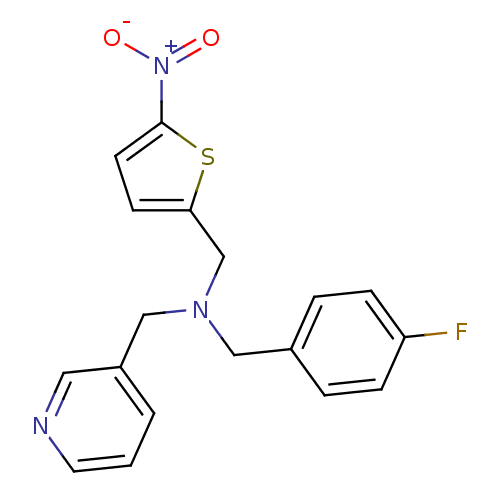
BDBM50434030 CHEMBL2381202
SMILES [O-][N+](=O)c1ccc(CN(Cc2ccc(F)cc2)Cc2cccnc2)s1
InChI Key InChIKey=HTLMWVUYXHRUOF-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50434030
Found 3 hits for monomerid = 50434030
TargetNuclear receptor subfamily 1 group D member 1(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataEC50: 160nMAssay Description:Agonist activity at biotinylated REV-ERBalpha (unknown origin) assessed as increase in biotinylated NCOR peptide recruitment after 1 hr by FRET assayMore data for this Ligand-Target Pair
TargetNuclear receptor subfamily 1 group D member 1(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataEC50: 160nMAssay Description:Agonist activity at Rev-Erb alpha (unknown origin) by FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+4nMAssay Description:Binding affinity to LXRalpha (unknown origin) by radioligand displacement assayMore data for this Ligand-Target Pair