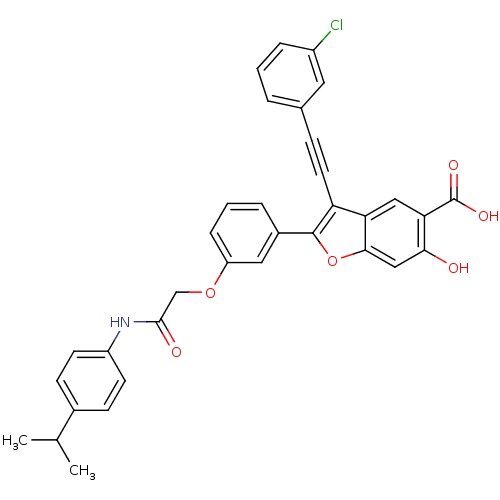
BDBM50436364 CHEMBL2396717
SMILES CC(C)c1ccc(NC(=O)COc2cccc(c2)-c2oc3cc(O)c(cc3c2C#Cc2cccc(Cl)c2)C(O)=O)cc1
InChI Key InChIKey=PPOLTQDGFSKQGI-UHFFFAOYSA-N
Data 2 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50436364
Found 2 hits for monomerid = 50436364
TargetTyrosine-protein phosphatase non-receptor type 22(Homo sapiens (Human))
Indiana University School Of Medicine
Curated by ChEMBL
Indiana University School Of Medicine
Curated by ChEMBL
Affinity DataIC50: 300nMAssay Description:Inhibition of N-terminal 6xHis-tagged LYP catalytic domain (1 to 294) (unknown origin) expressed in Escherichia coli BL21(DE3) using pNPP as substrat...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 22(Homo sapiens (Human))
Indiana University School Of Medicine
Curated by ChEMBL
Indiana University School Of Medicine
Curated by ChEMBL
Affinity DataIC50: 300nMAssay Description:Inhibition of N-terminal 6xHis-tagged LYP catalytic domain (1 to 303) (unknown origin) expressed in Escherichia coli BL21(DE3) using pNPP as substrat...More data for this Ligand-Target Pair