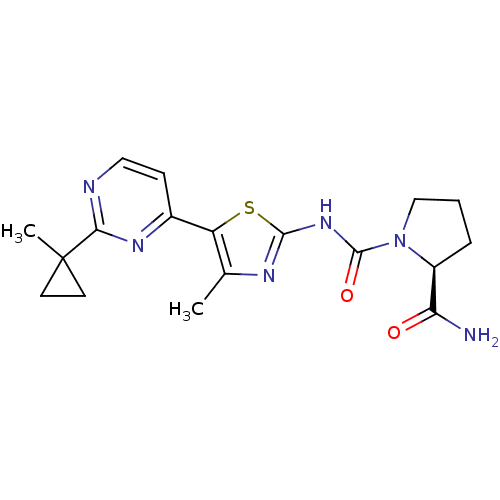
BDBM50436451 CHEMBL2397199
SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(n1)C1(C)CC1
InChI Key InChIKey=URGNTPNJBQTGQN-LBPRGKRZSA-N
Data 8 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 50436451
Found 8 hits for monomerid = 50436451
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 150nMAssay Description:Inhibition of N-terminal myristoylated P110alpha (unknown origin)-mediated AKT phosphorylation at Ser473 expressed in rat Rat1 cells by ELISAMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of N-terminal myristoylated P110delta (unknown origin)-mediated AKT phosphorylation at Ser473 expressed in rat Rat1 cells by ELISAMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 9.60E+3nMAssay Description:Inhibition of N-terminal myristoylated P110beta (unknown origin)-mediated AKT phosphorylation at Ser473 expressed in rat Rat1 cells by ELISAMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 810nMAssay Description:Inhibition of P110delta (unknown origin) using PIP2:PS as substrate by TR-FRET assayMore data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 3.10E+3nMAssay Description:Inhibition of P110beta (unknown origin) using L-a-phosphatidylinositol as substrate by luminescence assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 690nMAssay Description:Inhibition of P110gamma (unknown origin) using PIP2:PS as substrate by TR-FRET assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 12nMAssay Description:Inhibition of P110alpha (unknown origin) using L-a-phosphatidylinositol as substrate by luminescence assayMore data for this Ligand-Target Pair