BDBM50447023 CHEMBL1973869
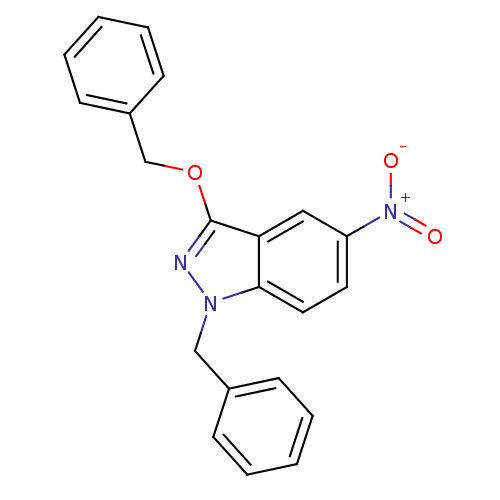
SMILES [O-][N+](=O)c1ccc2n(Cc3ccccc3)nc(OCc3ccccc3)c2c1
InChI Key InChIKey=RUJMERFUMDLBHZ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50447023
Found 3 hits for monomerid = 50447023
TargetCannabinoid receptor 2(Homo sapiens (Human))
Instituto De Qu£Mica M£Dica (Csic)
Curated by ChEMBL
Instituto De Qu£Mica M£Dica (Csic)
Curated by ChEMBL
Affinity DataKi: 400nMAssay Description:Displacement of [3H]CP55940 from human CB2R transfected in HEK293EBNA cell membrane incubated for 90 mins by Microbeta TriLux based luminescence anal...More data for this Ligand-Target Pair
TargetCannabinoid receptor 1(Homo sapiens (Human))
Instituto De Qu£Mica M£Dica (Csic)
Curated by ChEMBL
Instituto De Qu£Mica M£Dica (Csic)
Curated by ChEMBL
Affinity DataKi: 1.20E+3nMAssay Description:Displacement of [3H]CP55940 from human CB1R transfected in HEK293EBNA cell membranes incubated for 90 mins by Microbeta TriLux based luminescence ana...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of horse serum BuChE using butyrylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair