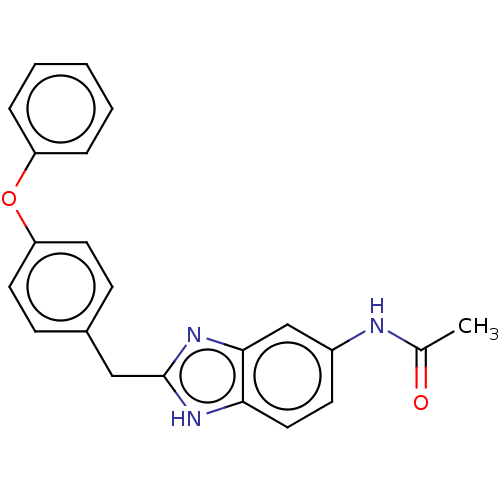
BDBM50474797 CHEMBL65245
SMILES CC(=O)Nc1ccc2[nH]c(Cc3ccc(Oc4ccccc4)cc3)nc2c1
InChI Key InChIKey=RCOPFJSNIYGOMZ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50474797
Found 3 hits for monomerid = 50474797
TargetGlutamate receptor ionotropic, NMDA 1/2B(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 93nMAssay Description:Compound was evaluated for in vitro inhibition of [3H][(E)-N-(2-methoxybenzyl)cinnamamidine binding to human NR1a/NR2B receptors expressed in LtK-cel...More data for this Ligand-Target Pair
TargetAlpha-1A/Alpha-1B/Alpha-1D adrenergic receptor(Rattus norvegicus (rat))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:In vitro inhibitory activity against alpha-1 adrenergic receptor binding to rat brain membranesMore data for this Ligand-Target Pair
TargetGlutamate receptor ionotropic, NMDA 1/2B(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 42nMAssay Description:In vitro inhibition of Glu/Gly stimulated [Ca2+] influx in LtK-cells expressing the hNR1a/NR2B receptorMore data for this Ligand-Target Pair