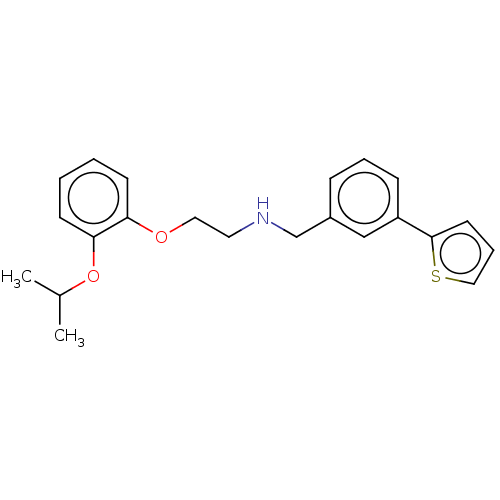
BDBM50476115 CHEMBL425810
SMILES CC(C)Oc1ccccc1OCCNCc1cccc(c1)-c1cccs1
InChI Key InChIKey=ZFRCVULPGRUJCI-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50476115
Found 4 hits for monomerid = 50476115
Affinity DataKi: 0.562nMAssay Description:Displacement of [3H]YM-09151-2 from rat striatum D2 receptorMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 1A(Rattus norvegicus (rat))
Pierre Fabre Research Center
Curated by ChEMBL
Pierre Fabre Research Center
Curated by ChEMBL
Affinity DataKi: 47nMAssay Description:Displacement of [3H]OH-DPAT from rat cortex 5HT1A receptorMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2A(Rattus norvegicus (rat))
Pierre Fabre Research Center
Curated by ChEMBL
Pierre Fabre Research Center
Curated by ChEMBL
Affinity DataKi: 562nMAssay Description:Displacement of [3H]ketanserin from rat cortex 5HT2A receptorMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 1A(Homo sapiens (Human))
Pierre Fabre Research Center
Curated by ChEMBL
Pierre Fabre Research Center
Curated by ChEMBL
Affinity DataEC50: <1.00E+4nMAssay Description:Agonist activity at human 5HT1A receptor in HeLa cells assessed as stimulation of [35S]GTP-gamma-S bindingMore data for this Ligand-Target Pair