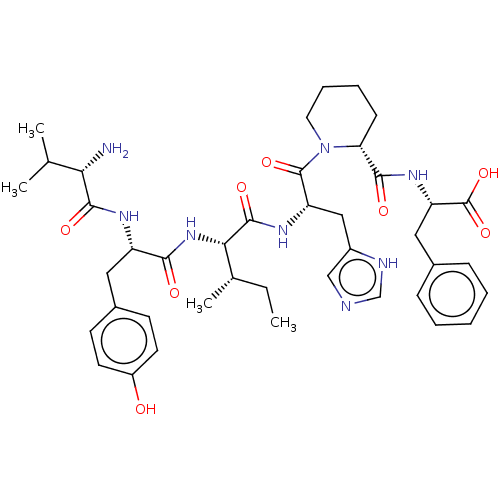
BDBM50478364 CHEMBL248592
SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](N)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCCC[C@@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O
InChI Key InChIKey=SQWZNTJOPGXAGA-RQCPJVKZSA-N
Data 2 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50478364
Found 2 hits for monomerid = 50478364
TargetLeucyl-cystinyl aminopeptidase(Homo sapiens (Human))
Vrije Universiteit Brussel
Curated by ChEMBL
Vrije Universiteit Brussel
Curated by ChEMBL
Affinity DataKi: 81nMAssay Description:Inhibition of human recombinant IRAP expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 2.95E+3nMAssay Description:Inhibition of human recombinant APN expressed in HEK293 cellsMore data for this Ligand-Target Pair