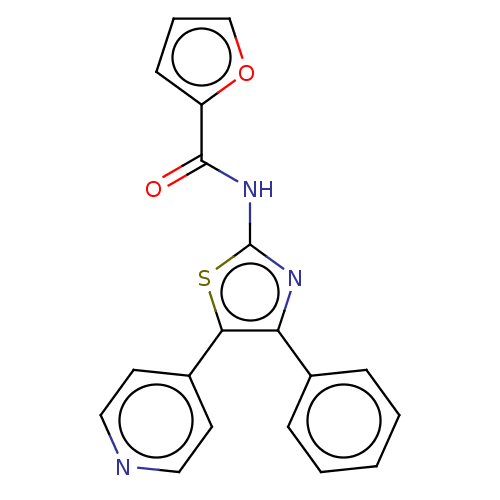
BDBM50492052 CHEMBL2391834
SMILES O=C(Nc1nc(c(s1)-c1ccncc1)-c1ccccc1)c1ccco1
InChI Key InChIKey=JYWRUTSZHRUZDL-UHFFFAOYSA-N
Data 8 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 50492052
Found 8 hits for monomerid = 50492052
TargetAdenosine receptor A1(Homo sapiens (Human))
B.V. Patel Pharmaceutical Education And Research Development
Curated by ChEMBL
B.V. Patel Pharmaceutical Education And Research Development
Curated by ChEMBL
Affinity DataKi: 0.580nMAssay Description:Displacement of [3H]CCPA from human adenosine A1 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetAdenosine receptor A1(Homo sapiens (Human))
B.V. Patel Pharmaceutical Education And Research Development
Curated by ChEMBL
B.V. Patel Pharmaceutical Education And Research Development
Curated by ChEMBL
Affinity DataKi: 0.580nMAssay Description:Displacement of [3H]CCPA from human adenosine receptor A1 expressed in CHO cell membranes incubated for 90 mins by radioligand competition assayMore data for this Ligand-Target Pair
TargetAdenosine receptor A2b(Homo sapiens (Human))
B.V. Patel Pharmaceutical Education And Research Development
Curated by ChEMBL
B.V. Patel Pharmaceutical Education And Research Development
Curated by ChEMBL
Affinity DataKi: 2.80nMAssay Description:Antagonist activity at human adenosine A2B receptor expressed in CHO cells assessed as inhibition of NECA-stimulated adenylyl cyclase activityMore data for this Ligand-Target Pair
TargetAdenosine receptor A2b(Homo sapiens (Human))
B.V. Patel Pharmaceutical Education And Research Development
Curated by ChEMBL
B.V. Patel Pharmaceutical Education And Research Development
Curated by ChEMBL
Affinity DataKi: 2.80nMAssay Description:Displacement of [3H]PSB-603 from human adenosine receptor A2B expressed in CHO cell membranes incubated for 75 mins by radioligand competition assayMore data for this Ligand-Target Pair
Affinity DataKi: 6.70nMAssay Description:Displacement of [3H]MSX2 from human adenosine receptor A2A expressed in HEK293 cell membranes incubated for 30 mins by radioligand competition assayMore data for this Ligand-Target Pair
Affinity DataKi: 6.70nMAssay Description:Displacement of [3H]NECA from human adenosine A2A receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 9.40nMAssay Description:Displacement of [3H]PSB-11 from human adenosine receptor A3 expressed in CHO cell membranes incubated for 60 mins by radioligand competition assayMore data for this Ligand-Target Pair
Affinity DataKi: 9.40nMAssay Description:Displacement of [3H]HEMADO from human adenosine A3 receptor expressed in CHO cellsMore data for this Ligand-Target Pair