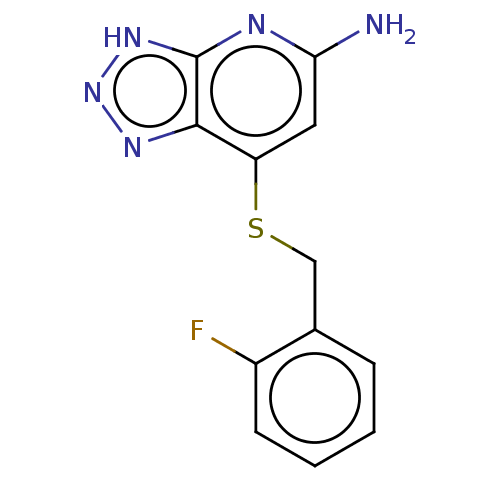
BDBM50507390 CHEMBL4482878::US10981879, Example 3
SMILES Nc1cc(SCc2ccccc2F)c2nn[nH]c2n1
InChI Key InChIKey=JMLWUWYLBNAXLU-UHFFFAOYSA-N
Data 7 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 7 hits for monomerid = 50507390
Found 7 hits for monomerid = 50507390
TargetMyeloperoxidase(Homo sapiens (Human))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataIC50: 6.80nMAssay Description:Inhibition of human PMN leukocytes MPO chlorination activity using H2O2 as substrate preincubated for 10 mins followed by H2O2 addition and measured ...More data for this Ligand-Target Pair
TargetLactoperoxidase(Homo sapiens (Human))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataIC50: >8.00E+3nMAssay Description:Inhibition of human LPO assessed as reduction in H2O2 catalyzed 3,5-iodo tyrosine formation from 3-iodotyrosine and potassium iodide preincubated for...More data for this Ligand-Target Pair
Affinity DataIC50: 22nMAssay Description:Inhibition of human EPX bromination activity assessed as reduction in H2O2 catalyzed 3-bromo tyrosine formation from tyrosine and potassium bromide p...More data for this Ligand-Target Pair
TargetMyeloperoxidase(Homo sapiens (Human))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataIC50: 7nMAssay Description:MPO peroxidation activity was measured in 100 mM KPi (pH 7.4) by utilizing the non-fluorescent reagent Amplex Red (Invitrogen catalog #A12222) which ...More data for this Ligand-Target Pair
TargetMyeloperoxidase(Homo sapiens (Human))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataIC50: 8.70nMAssay Description:Inhibition of human PMN leukocytes MPO peroxidation activity using H2O2 as substrate preincubated for 10 mins followed by H2O2 addition and measured ...More data for this Ligand-Target Pair
TargetMyeloperoxidase(Homo sapiens (Human))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:MPO chlorination activity was measured in 100 mM KPi (pH 7.4) by utilizing the non-fluorescent reagent Aminophenyl fluorescein (APF, Invitrogen catal...More data for this Ligand-Target Pair
TargetThyroid peroxidase(Homo sapiens (Human))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataIC50: 4.00E+3nMAssay Description:Inhibition of human TPO assessed as reduction in H2O2 catalyzed 3,5-iodo tyrosine formation from 3-iodotyrosine and potassium iodide preincubated for...More data for this Ligand-Target Pair