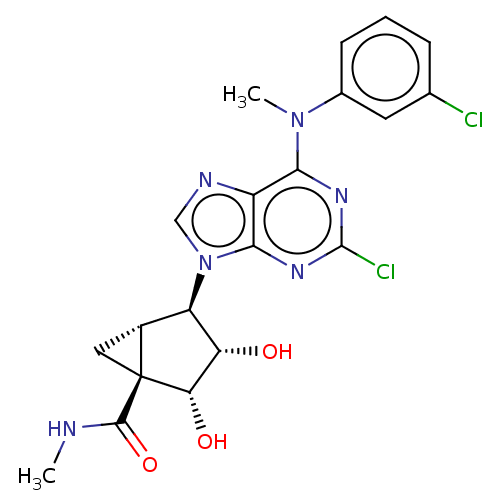
BDBM50518050 CHEMBL4464176
SMILES [H][C@]12C[C@]1([C@@H](O)[C@@H](O)[C@@H]2n1cnc2c(nc(Cl)nc12)N(C)c1cccc(Cl)c1)C(=O)NC
InChI Key InChIKey=WBUOCDGSBFEIOR-DDDALXFXSA-N
Data 3 EC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50518050
Found 3 hits for monomerid = 50518050
Affinity DataEC50: 0.251nMAssay Description:Agonist activity at human adenosine A3 receptor expressed in FlpIn-CHO cells assessed as inhibition of forskolin-mediated cAMP accumulation preincuba...More data for this Ligand-Target Pair
Affinity DataEC50: 0.158nMAssay Description:Agonist activity at human adenosine A3 receptor expressed in FlpIn-CHO cells assessed as increase in ERK1/2 phosphorylation after 5 mins by alphascre...More data for this Ligand-Target Pair
Affinity DataEC50: 0.0631nMAssay Description:Agonist activity at adenosine A3 receptor (unknown origin) expressed in serum starved CHO cells assessed as increase in cell survival after 24 hrs by...More data for this Ligand-Target Pair