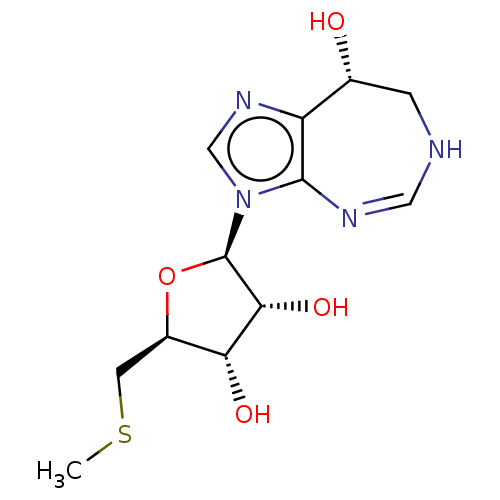
BDBM50519494 CHEMBL1234234
SMILES CSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12
InChI Key InChIKey=QLPPCUVJNCMYFD-SANHVUMCSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50519494
Found 3 hits for monomerid = 50519494
Affinity DataKi: 0.430nMAssay Description:Inhibition of Plasmodium falciparum N-terminal thrombin cleavable His6-tagged ADA expressed in Escherichia coli BL21 assessed as equilibrium dissocia...More data for this Ligand-Target Pair
Affinity DataKi: 2.70nMAssay Description:Inhibition of Plasmodium falciparum N-terminal thrombin cleavable His6-tagged ADA expressed in Escherichia coli BL21 assessed as reduction in formati...More data for this Ligand-Target Pair
Affinity DataKi: >10nMAssay Description:Inhibition of human erythrocytes ADA assessed as reduction in formation of inosine using adenosine as substrateMore data for this Ligand-Target Pair