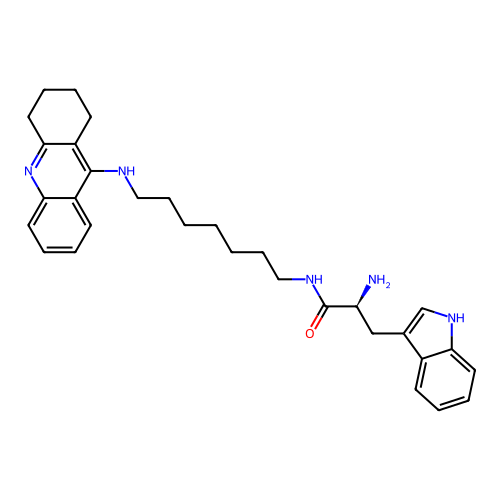
BDBM50523389 CHEMBL4559477
SMILES Cl.Cl.N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCCCCCCCNc1c2CCCCc2nc2ccccc12
InChI Key InChIKey=NSHWMFUZUABTHF-ROPHLPQBSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50523389
Found 3 hits for monomerid = 50523389
Affinity DataIC50: 25nMAssay Description:Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured...More data for this Ligand-Target Pair
TargetNitric oxide synthase, endothelial(Rattus norvegicus)
University Of Hradec Kralove
Curated by ChEMBL
University Of Hradec Kralove
Curated by ChEMBL
Affinity DataIC50: 3.20E+4nMAssay Description:Inhibition of nNOS in Wistar rat cortical homogenates incubated for 30 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 120nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured ...More data for this Ligand-Target Pair