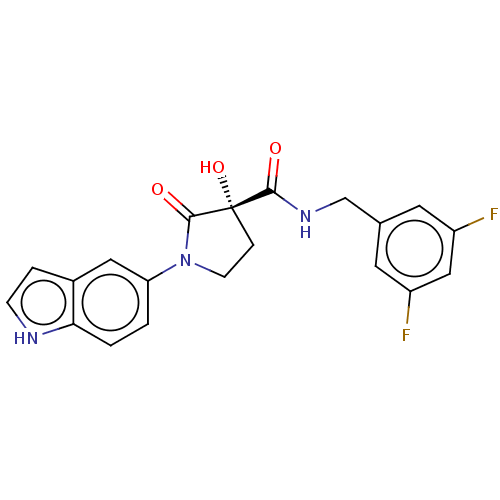
BDBM50531163 CHEMBL4464946
SMILES O[C@@]1(CCN(C1=O)c1ccc2[nH]ccc2c1)C(=O)NCc1cc(F)cc(F)c1
InChI Key InChIKey=WVGGJQVCOTYFPV-FQEVSTJZSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 10 hits for monomerid = 50531163
Found 10 hits for monomerid = 50531163
Affinity DataKi: 4.30nMAssay Description:Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b...More data for this Ligand-Target Pair
Affinity DataKi: 4.30nMAssay Description:Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b...More data for this Ligand-Target Pair
Affinity DataKd: 2.10nMAssay Description:Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b...More data for this Ligand-Target Pair
Affinity DataIC50: 54nMAssay Description:Inhibition of recombinant human N-terminal His-tagged MetAP2 (2 to 478 residues) using Met-Ala-Ser as substrate and MnCl2 as co-facor preincubated fo...More data for this Ligand-Target Pair
Affinity DataIC50: 54nMAssay Description:Inhibition of recombinant human N-terminal His-tagged MetAP2 (2 to 478 residues) using Met-Ala-Ser as substrate and MnCl2 as co-facor preincubated fo...More data for this Ligand-Target Pair
Affinity DataKd: 2.10nMAssay Description:Inhibition of 5-[(S)-3-(3-Chloro-5-fluoro-benzylcarbamoyl)-3-hydroxy-2-oxopyrrolidin-1-yl]-1H-indole-2-carboxylic Acid (3-Amino-propyl)-amide-Dy647 b...More data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:Inhibition of MetAP2 in HUVEC assessed as reduction in viability incubated for 3 days by CyQUANT Direct Cell proliferation assayMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of N-terminal MetAP1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:Inhibition of MetAP2 in HUVEC assessed as reduction in viability incubated for 3 days by CyQUANT Direct Cell proliferation assayMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of N-terminal MetAP1 (unknown origin)More data for this Ligand-Target Pair