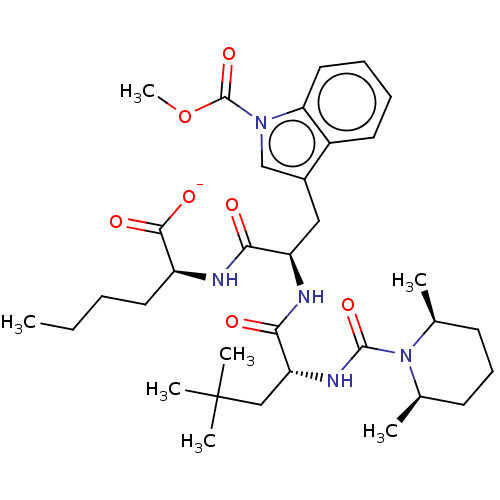
BDBM50534382 CHEMBL4568795
SMILES [Na;v0+].[#6]-[#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-c1cn(-[#6](=O)-[#8]-[#6])c2ccccc12)-[#7]-[#6](=O)-[#6@@H](-[#6]C([#6])([#6])[#6])-[#7]-[#6](=O)-[#7]-1-[#6@@H](-[#6])-[#6]-[#6]-[#6]-[#6@H]-1-[#6])-[#6](-[#8-])=O
InChI Key InChIKey=QCVIFBRTTLMEOV-YPKROFLLSA-M
Data 2 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50534382
Found 2 hits for monomerid = 50534382
TargetEndothelin receptor type B(Homo sapiens (Human))
Centre Hospitalier Universitaire Vaudois (Chuv)
Curated by ChEMBL
Centre Hospitalier Universitaire Vaudois (Chuv)
Curated by ChEMBL
Affinity DataIC50: 1.20nMAssay Description:Displacement of [125I]ET-1 from ET-B receptor in human Girardi heart cells incubated for 4 hrs by gamma counting methodMore data for this Ligand-Target Pair
TargetEndothelin-1 receptor(Homo sapiens (Human))
Centre Hospitalier Universitaire Vaudois (Chuv)
Curated by ChEMBL
Centre Hospitalier Universitaire Vaudois (Chuv)
Curated by ChEMBL
Affinity DataIC50: 1.30E+3nMAssay Description:Displacement of [125I]ET-1 from ET-A receptor in human SK-N-MC cells incubated for 4 hrs by gamma counting methodMore data for this Ligand-Target Pair