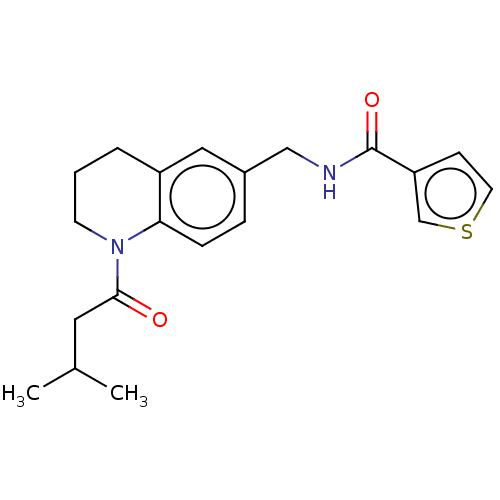
BDBM50552924 CHEMBL4788767
SMILES CC(C)CC(=O)N1CCCc2cc(CNC(=O)c3ccsc3)ccc12
InChI Key InChIKey=GMEPSDDJIZYOME-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50552924
Found 3 hits for monomerid = 50552924
Affinity DataIC50: 94nMAssay Description:Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.25E+3nMAssay Description:Inhibition of IDO1 in LPS and IFN-gamma-stimulated human Whole blood assessed as reduction in kynurenine production using isotope-labeled tryptophan ...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck
Curated by ChEMBL
Merck
Curated by ChEMBL
Affinity DataIC50: 2.40E+4nMAssay Description:Displacement of [35S]-MK499 binding to human ERG expressed in HEK293 cells incubated for 2 hrs by liquid scintillation counting methodMore data for this Ligand-Target Pair